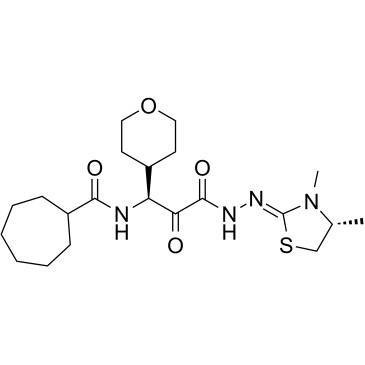
ONO-5334 構造式
CAS 番号 : 868273-90-9

COVID-19:小野薬品の骨粗鬆症薬が有望:ONO 5334(動画):
COVID-19: Promising osteoporosis drug from Ono:ONO 5334:
COVID-19:来自Ono的有前途的骨质疏松症药物:ONO 5334
COVID-19:
【ワシントン】
米国チームが24日、英科学誌ネイチャーに発表しました。
細胞実験の調査結果:
開発中や既存の薬約1万2千種類について、COVID-19治療に役立つかどうかを、細胞実験で調べました。
結果、米国のチームが、7月24日「3種類が有望とする論文」を英科学誌ネイチャーで発表しました。
ONO5334:
そのうち一つは、小野薬品工業(大阪)が、骨粗しょう症治療のために開発したが、実用化を中止した「ONO5334」という薬です。
小野薬品は「内容を精査して対応を検討する」とのこと。
肺の培養細胞でウイルス感染実験:
今回の実験は、「人のiPS細胞からつくった肺の培養細胞を、ウイルスに感染させる実験」です。
ONO5334を投与した場合に感染細胞数が72%減少。
他の2種類も、65~85%減少したという。
共同通信
https://this.kiji.is/659573789646046305
Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing
Published: 24 July 2020
This is an unedited manuscript that has been accepted for publication.
Nature Research
are providing this early version of the manuscript as a service to our authors and readers.
The manuscript will undergo copyediting, typesetting and a proof review before it is published in its final form.
Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers apply.
Laura Riva, Shuofeng Yuan, […]Sumit K. Chanda
Abstract
The emergence of the novel SARS coronavirus 2 (SARS-CoV-2) in 2019
has triggered an ongoing global pandemic of severe pneumonia-like disease designated as coronavirus disease 2019 (COVID-19)1.
The development of a vaccine
is likely to require at least 12-18 months, and the typical timeline for approval of a novel antiviral therapeutic can exceed 10 years.
Thus, repurposing of known drugs
could significantly accelerate the deployment of novel therapies for COVID-19.
Towards this end, we profiled a library of known drugs encompassing approximately 12,000 clinical-stage or FDA-approved small molecules.
We report the identification of 100 molecules that inhibit viral replication, including 21 known drugs that exhibit dose response relationships.
Of these, thirteen
were found to harbor effective concentrations likely commensurate with achievable therapeutic doses in patients,
including
- the PIKfyve kinase inhibitor apilimod2–4,
- the cysteine protease inhibitors
- MDL-28170,
- Z LVG CHN2,
- VBY-825,
- ONO 5334.
Notably,
- MDL-28170,
- ONO 5334,
- apilimod
were found to antagonize viral replication in human iPSC-derived pneumocyte-like cells,
the PIKfyve inhibitor also
demonstrated antiviral efficacy in a primary human lung explant model.
Since most of the molecules identified in this study have already advanced into the clinic, the known pharmacological
and human safety profiles of these compounds will enable accelerated preclinical and clinical evaluation of these drugs for the treatment of COVID-19.
https://www.nature.com/articles/s41586-020-2577-1
ONO-5334 | Cathepsin K 阻害剤 |
ONO-5334 is a potent, selective and orally active cathepsin K inhibitor with Ki values of 0.10 nM, 0.049 nM and 0.85 nM for human, rabbit and rat cathepsin K, respectively. ONO-5334 has the potential for the study of osteoporosis.
MedChemExpress