COVID-19:「5-ALA」は変異株にも有効:長崎大が発表(動画):
COVID-19: “5-ALA” is also effective for mutant strains: Nagasaki Univ:
COVID-19:“5-ALA”对突变菌株也有效:长崎大学宣布
ーコロナ治療薬の研究ー
長崎大
製薬会社ネオファーマジャパン(東京)
- 新型コロナウイルスの感染症治療薬として、
- 「5-アミノレブリン酸」について、
- 合同で臨床研究中。
1月11日、
「デルタ株など4種類の変異株に対しても、ウイルス増殖抑制効果がある」と発表した。
熱帯医学・グローバルヘルス研究科長の北潔教授らが研究。
国際学術誌の電子版に7日、掲載された。
「5-アミノレブリン酸」:
「5-ALA」はヒトをはじめ、動植物の細胞内で合成されるアミノ酸。
- ミトコンドリアの活性化や抗酸化作用、
- 免疫力向上などの機能があり、
- 健康食品や抗がん治療に活用されている。
北教授らの研究:
新型コロナ感染症の予防や治療、後遺症治療を目指している。
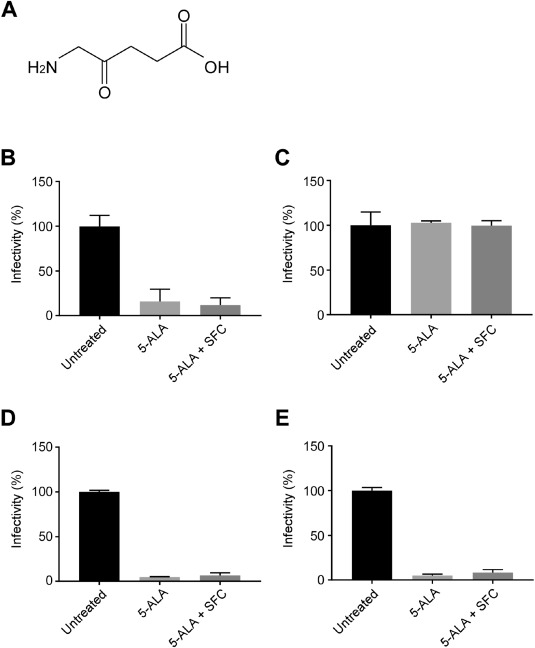
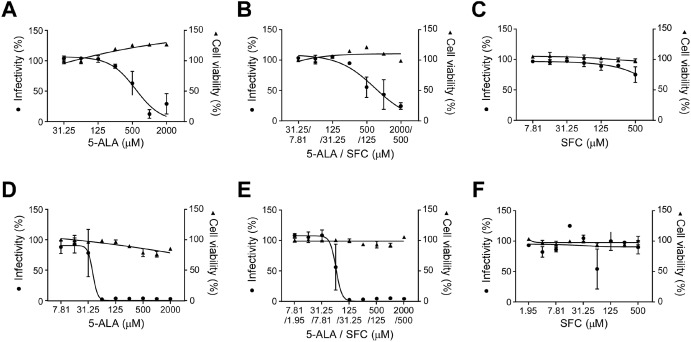
これまで従来株の感染抑制効果を確認した。
今回新たに、
アルファ、ベータ、ガンマ、デルタ株で試験管実験を行い、
一定濃度以上であれば、同様の効果があると突き止めた。
現在、感染急拡大中のオミクロン株に対する実験も準備している。
臨床試験を昨年実施:
昨年、長崎大学病院など県内外8病院。
新型コロナ軽症や中等症患者50人に、「5-ALA」を投与した。
既にデータ解析を終え、3月末までに研究結果を公表したい。
長崎新聞
https://nordot.app/853820171904483328
5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro – ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0006291X2100156X
Neopharma Japan Begins Specific Clinical Trials Using 5-Aminolevulinic Acid (5-ALA) on Novel Coronavirus (COVID-19) Patients at Nagasaki University
Nagasaki University,
in association with neopharma Japan Co., Ltd. (as “NPJ”)
today announced that
they have begun specific clinical trials using 5-aminolevulinic acid *3 (hereinafter referred to as ‘5-ALA’) researched, developed and manufactured by the company, on patients infected with the novel coronavirus (hereinafter referred to as ’COVID-19’).
5-ALA is a naturally occurring amino acid
that is produced within the cells of humans, animals and plants and animals.
It is also contained in foods
and is one of the amino acids that we ingest as part of our daily lives.
Furthermore,
due to its tremendous safety and functionality, it has already been utilized in various healthcare products for more than ten years.
NPJ, in association with Prof. Kiyoshi Kita, Dean of the School of Tropical Medicine and Global Health, Nagasaki University,
has focused on this functionality of 5-ALA and has been developing therapeutic agents for malaria.
Currently we are conducting clinical research in Laos
in association with Director Shigeyuki Kano of the National Center for Global Health and Medicine.
As 5-ALA is expected to be effective against a wide range of infectious diseases,
we are extensively studying the inhibitory effects of 5-ALA against tropical infectious diseases in association with Nagasaki University, which has a long history of research into tropical diseases.
Against this backdrop, Nagasaki Uni.
has begun research on COVID-19 in response to the high societal demand.
From the results of an in-vitro cell infection test conducted with the usage of the causative virus, SARS-CoV-2,
we discovered that 5-ALA possessed a strong infection-suppressing effect against the infection.
The results of this research have been submitted as a paper. *4
Professor Koichi Izumikawa,
Vice President of Nagasaki Uni. (COVID-19 Response) and others have drafted a specific clinical trial for the administration of 5-ALA to patients infected with COVID-19,
and as 28/10/2020,
this specific clinical trial was officially approved by the Accredited Ethics Committee of Nagasaki Uni.
The detailed contents of this specific clinical trial will be published in the clinical study database at a later date.
This is a multi-institutional joint study conducted at multiple hospitals, with Nagasaki Uni. Hospital functioning as the core.
Nagasaki University
https://www.nagasaki-u.ac.jp/en/news/news69.html