COVID-19:“5-ALA”对突变菌株也有效:长崎大学宣布
-关于电晕疗法的研究-
长崎大学
制药公司 Neopharma Japan(东京)
作为新型冠状病毒感染性疾病的治疗方法
关于“5-氨基乙酰丙酸”
正在进行联合临床研究。
1月11日,
“它还对四种类型的突变株如Delta株具有病毒生长抑制作用,”他说。它是
由热带医学与全球健康研究生院院长 Kiyoshi Kita 教授研究。
7日发表在国际学术期刊电子版上。
“5-氨基乙酰丙酸”:
“5-ALA”是在包括人类在内的动植物细胞中合成的氨基酸。
线粒体活化和抗氧化活性,
具有提高免疫力、
用于保健食品和抗癌治疗。
Kita教授等人的研究:
我们的目标是预防和治疗新的冠状病毒感染和治疗后遗症。
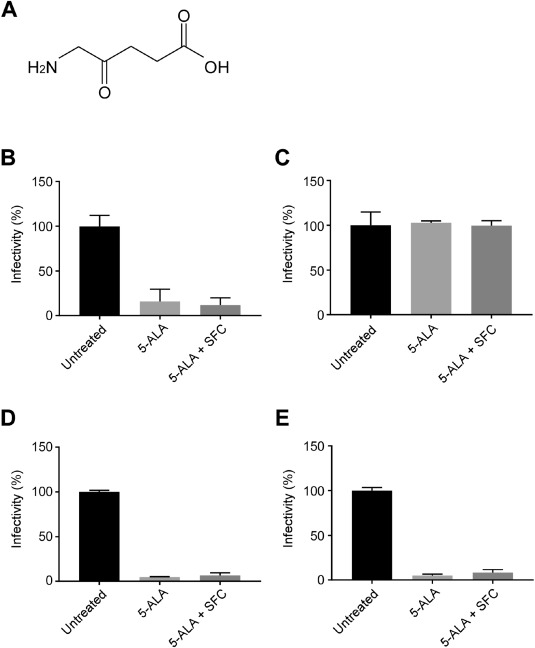
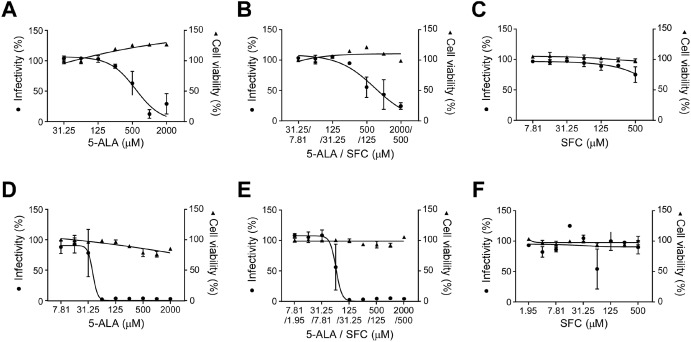
到目前为止,我们已经证实了常规菌株的感染抑制作用。
这次新
α、β、γ和δ菌株的试管实验
结果发现,如果浓度高于一定水平,则获得相同的效果。
目前,我们正在准备对正在迅速传播的 Omicron 菌株进行实验。
去年进行的临床试验:
去年,包括长崎大学医院在内的县内外有8家医院。
50 名轻度或中度电晕患者接受了“5-ALA”。
我已经完成了数据分析,想在三月底前公布研究结果。
长崎新闻
https://nordot.app/853820171904483328
5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro – ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0006291X2100156X
Neopharma Japan Begins Specific Clinical Trials Using 5-Aminolevulinic Acid (5-ALA) on Novel Coronavirus (COVID-19) Patients at Nagasaki University
Nagasaki University,
in association with neopharma Japan Co., Ltd. (as “NPJ”)
today announced that
they have begun specific clinical trials using 5-aminolevulinic acid *3 (hereinafter referred to as ‘5-ALA’) researched, developed and manufactured by the company, on patients infected with the novel coronavirus (hereinafter referred to as ’COVID-19’).
5-ALA is a naturally occurring amino acid
that is produced within the cells of humans, animals and plants and animals.
It is also contained in foods
and is one of the amino acids that we ingest as part of our daily lives.
Furthermore,
due to its tremendous safety and functionality, it has already been utilized in various healthcare products for more than ten years.
NPJ, in association with Prof. Kiyoshi Kita, Dean of the School of Tropical Medicine and Global Health, Nagasaki University,
has focused on this functionality of 5-ALA and has been developing therapeutic agents for malaria.
Currently we are conducting clinical research in Laos
in association with Director Shigeyuki Kano of the National Center for Global Health and Medicine.
As 5-ALA is expected to be effective against a wide range of infectious diseases,
we are extensively studying the inhibitory effects of 5-ALA against tropical infectious diseases in association with Nagasaki University, which has a long history of research into tropical diseases.
Against this backdrop, Nagasaki Uni.
has begun research on COVID-19 in response to the high societal demand.
From the results of an in-vitro cell infection test conducted with the usage of the causative virus, SARS-CoV-2,
we discovered that 5-ALA possessed a strong infection-suppressing effect against the infection.
The results of this research have been submitted as a paper. *4
Professor Koichi Izumikawa,
Vice President of Nagasaki Uni. (COVID-19 Response) and others have drafted a specific clinical trial for the administration of 5-ALA to patients infected with COVID-19,
and as 28/10/2020,
this specific clinical trial was officially approved by the Accredited Ethics Committee of Nagasaki Uni.
The detailed contents of this specific clinical trial will be published in the clinical study database at a later date.
This is a multi-institutional joint study conducted at multiple hospitals, with Nagasaki Uni. Hospital functioning as the core.
Nagasaki University
https://www.nagasaki-u.ac.jp/en/news/news69.html