COVID-19: “5-ALA” is also effective for mutant strains: Nagasaki University announced
-Research on corona remedies-
Nagasaki University
Pharmaceutical company Neopharma Japan (Tokyo)
As a treatment for infectious diseases of the new coronavirus
About “5-aminolevulinic acid”
Under joint clinical research.
January 11th,
“It also has a virus growth inhibitory effect on four types of mutant strains such as Delta strains,” he said. It was
Researched by Professor Kiyoshi Kita, Dean of the Graduate School of Tropical Medicine and Global Health.
It was published in the electronic version of an international academic journal on the 7th.
“5-Aminolevulinic acid”:
“5-ALA” is an amino acid synthesized in the cells of animals and plants including humans.
Mitochondrial activation and antioxidant activity,
It has functions such as improving immunity,
It is used for health foods and anti-cancer treatment.
Research by Professor Kita et al .:
We aim to prevent and treat new corona infections and treat sequelae.
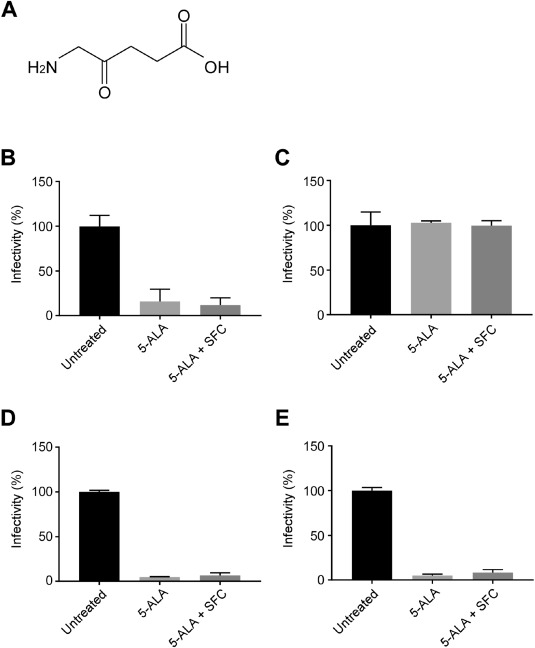
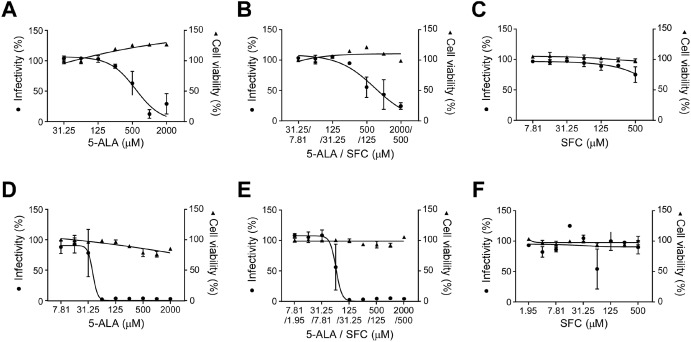
So far, we have confirmed the infection-suppressing effect of conventional strains.
This time newly
Test tube experiments with alpha, beta, gamma, and delta strains
It was found that the same effect was obtained if the concentration was above a certain level.
Currently, we are preparing an experiment for the Omicron strain, which is rapidly spreading.
Conducted clinical trials last year:
Last year, there were 8 hospitals inside and outside the prefecture, including Nagasaki University Hospital.
“5-ALA” was administered to 50 patients with mild or moderate corona.
I have already completed the data analysis and would like to publish the research results by the end of March.
Nagasaki Shimbun
https://nordot.app/853820171904483328
5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro – ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0006291X2100156X
Neopharma Japan Begins Specific Clinical Trials Using 5-Aminolevulinic Acid (5-ALA) on Novel Coronavirus (COVID-19) Patients at Nagasaki University
Nagasaki University,
in association with neopharma Japan Co., Ltd. (as “NPJ”)
today announced that
they have begun specific clinical trials using 5-aminolevulinic acid *3 (hereinafter referred to as ‘5-ALA’) researched, developed and manufactured by the company, on patients infected with the novel coronavirus (hereinafter referred to as ’COVID-19’).
5-ALA is a naturally occurring amino acid
that is produced within the cells of humans, animals and plants and animals.
It is also contained in foods
and is one of the amino acids that we ingest as part of our daily lives.
Furthermore,
due to its tremendous safety and functionality, it has already been utilized in various healthcare products for more than ten years.
NPJ, in association with Prof. Kiyoshi Kita, Dean of the School of Tropical Medicine and Global Health, Nagasaki University,
has focused on this functionality of 5-ALA and has been developing therapeutic agents for malaria.
Currently we are conducting clinical research in Laos
in association with Director Shigeyuki Kano of the National Center for Global Health and Medicine.
As 5-ALA is expected to be effective against a wide range of infectious diseases,
we are extensively studying the inhibitory effects of 5-ALA against tropical infectious diseases in association with Nagasaki University, which has a long history of research into tropical diseases.
Against this backdrop, Nagasaki Uni.
has begun research on COVID-19 in response to the high societal demand.
From the results of an in-vitro cell infection test conducted with the usage of the causative virus, SARS-CoV-2,
we discovered that 5-ALA possessed a strong infection-suppressing effect against the infection.
The results of this research have been submitted as a paper. *4
Professor Koichi Izumikawa,
Vice President of Nagasaki Uni. (COVID-19 Response) and others have drafted a specific clinical trial for the administration of 5-ALA to patients infected with COVID-19,
and as 28/10/2020,
this specific clinical trial was officially approved by the Accredited Ethics Committee of Nagasaki Uni.
The detailed contents of this specific clinical trial will be published in the clinical study database at a later date.
This is a multi-institutional joint study conducted at multiple hospitals, with Nagasaki Uni. Hospital functioning as the core.
Nagasaki University
https://www.nagasaki-u.ac.jp/en/news/news69.html