
Parkinson’s disease: iPS cell transplantation begins in the US
– Japanese-made IPS cells to be transplanted into US patients
– Parkinson’s disease treatment using iPS cells begins
Summary from Kansai NHK NEWS article.
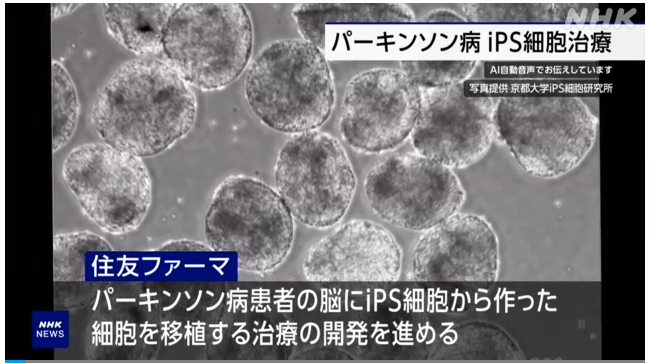
Treatment of Parkinson’s disease with iPS cells:
Treatment of Parkinson’s disease using iPS cells produced by an Osaka pharmaceutical company.
1. On June 25th, iPS cells were transplanted into the first patient in a clinical trial in the US.
2. iPS cell transplantation has already been successful in a clinical trial in Japan, with good results.
The Japanese company is preparing to apply for manufacturing and sales approval, and will work to commercialize the treatment overseas.
。
Pharmaceutical company “Sumitomo Pharma”:
Developed a treatment to transplant cells created from iPS cells into the brains of Parkinson’s disease patients.
Aiming to commercialize the treatment overseas. Last year, the company announced that it would conduct clinical trials in the US.

University Hospital in California: Conducting iPS Clinical Trials:
In June of this year, a facility in Osaka succeeded in “packing and transporting live iPS cells created in Japan.”
1. The iPS cells were provided for clinical trials at the University of California, San Diego, via a 23-hour flight.
2. On June 25th, Japan time, the first case of the clinical trial was transplanted into a patient in the United States.
The iPS clinical trials in Japan have already been successful, and the company will now apply for government approval to manufacture and sell the cells.
What is Parkinson’s disease:
An incurable disease in which the brain’s nerve cells involved in movement are reduced, causing symptoms such as tremors and stiffness.
In 2018, Kyoto University in Japan began clinical trials to inject nerve cells created from other people’s iPS cells into the brain.
Sumitomo Pharma is responsible for manufacturing the cells and is preparing to apply for approval in Japan.
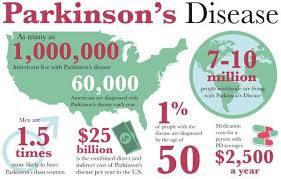
Number of Parkinson’s disease patients in the United States:
1. The number of patients in the US is more than three times that of Japan, at approximately 1 million.
2. The practical application of iPS cell therapy is attracting attention overseas, where the market size is large.
Sumitomo Pharma aims to apply for approval in the US.
Yoshida Kenji, Director, Sumitomo Pharma Regenerative Medicine and Cellular Medicine Promotion Office
1. Seven patients are planned to be treated in this US clinical trial.
2. Clinical trials are planned for even more patients.
We would like to develop this treatment together with many stakeholders and deliver it to patients around the world as soon as possible.
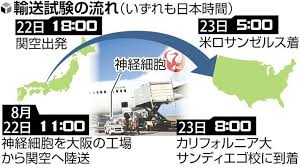
Ideas for shortening transportation time:
Sumitomo Pharma produced iPS cells at its own factory in Suita City, Osaka Prefecture, and delivered them to a hospital at the University of California, San Diego.
1. In fact, “iPS cells need to be transported alive.” They are airlifted alive at a temperature of 2 to 8 degrees.
2. The goal is to deliver them within 24 hours of shipping to prevent deterioration of quality.

Time lost in customs procedures: Aiming for less than 24 hours
Sumitomo Pharma began testing international air transport of iPS cells in 2020.
1. However, after arriving at Los Angeles Airport, customs procedures took a long time.
2. Local customs procedures in the United States apparently took about 40 to 60 hours.
In the final test on a scheduled flight from Kansai Airport to Los Angeles, the target was achieved in a total of 21 hours and 14 minutes.

Cooperation with Japan Airlines and others: Utilization of “bulk cargo hold”:
In order to deliver live iPS cells in good condition, Mitsubishi Warehouse, Inabata & Co., Ltd., and Japan Airlines have teamed up.
1. Only the iPS cells are loaded into the “bulk cargo hold” and customs procedures are quickly carried out after arrival.
2. In this transport, the cells were delivered to the local university hospital in 23 hours.
https://www3.nhk.or.jp/kansai-news/20250707/2000095108.html