
Parkinson’s disease: iPS cell transplants restore motor function
・Kyoto University transplants iPS cells into seven patients, confirming safety and effectiveness
・Nerve cells that produce dopamine from iPS cells are transplanted into patients’ brains
Summary from an article published on NHK.
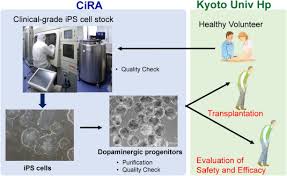
Kyoto University: Successful treatment for Parkinson’s disease
A new treatment has been developed in which iPS cells are transplanted into the brains of Parkinson’s disease patients.
1. A research team from Kyoto University conducted clinical trials on seven patients.
2. It was announced that the safety and effectiveness of human iPS cell transplants has been proven.
Pharmaceutical company that cooperated in the clinical trial: Sumitomo Pharma
Sumitomo Pharma, an Osaka-based pharmaceutical company that cooperated in the clinical trial, will apply to the government for manufacturing and sales based on this data.
1. It is revolutionary for researchers that symptoms can be improved by cell transplants.
2. We hope to obtain government approval and deliver the treatment to patients as soon as possible.
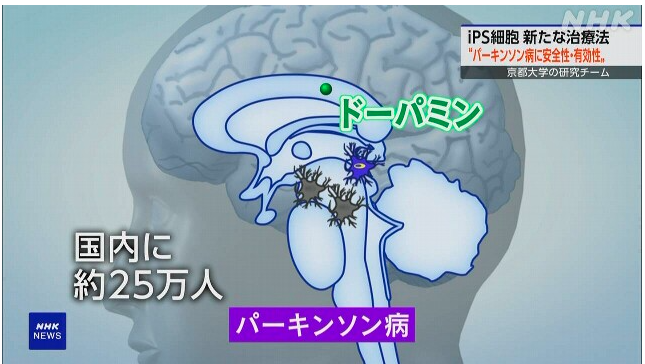
What is Parkinson’s disease:
A disease in which brain cells that produce the neurotransmitter “dopamine” are lost.
1. It is an incurable disease that causes tremors in the limbs and immobility.
2. There are 250,000 patients in Japan.
3. Treatment is mainly through the administration of medicine and implantation of electrodes in the brain.
At present, no fundamental treatment has been found anywhere in the world.
Kyoto University Center for iPS Cell Research and Application: Professor Jun Takahashi et al.:
1. Mass-produce “dopamine-producing nerve cells” from human iPS cells.
2. After that, transplant the iPS cells into the patient’s brain to improve symptoms.
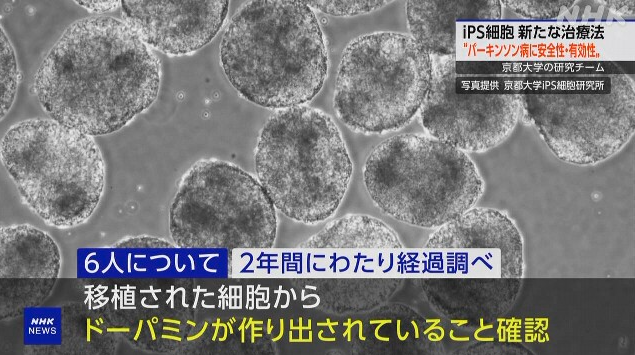
Details of this clinical trial:
1. In the clinical trial, 10 million cells were transplanted into the brains of seven male and female patients aged 50 to 69.
2. No major health problems were observed in any of the patients.
3. The progress of six of these patients was monitored over a two-year period.
4. In both patients, it was confirmed that “dopamine was produced from the transplanted cells.”
In symptom recovery tests, motor function improved in four out of six patients.

Wife of a Parkinson’s patient: 66-year-old woman:
1. It has been many years since my husband was diagnosed with the disease, and he has been falling more often and having difficulty speaking.
2. The success of the iPS cell treatment is a new hope for the family.

National Parkinson’s Disease Association: Kyoto Prefecture Chapter Chief Okada Takashi
1. It’s like a dream. It’s a groundbreaking treatment, and I hope it will be implemented soon.
2. I’ve heard that it is more effective for younger people.
I hope that it will become an effective treatment for all patients.

Juntendo University: Special Professor Hattori Nobutaka:
The results of this clinical trial will provide a new option for Parkinson’s disease treatment.
1. It may be possible to “reduce the number of times you take medication or even eliminate it altogether.”
2. For patients, this improves their condition while sleeping and reduces the risk of forgetting to take the drug.
It will be necessary to verify in the future what types of patients this drug is effective for.
Improvement of patients’ motor function:
This clinical trial was conducted on seven patients.
1. For one patient, only safety was confirmed.
2. Six patients were examined for the effectiveness of Parkinson’s disease treatment.
The younger the patient, the more effective the treatment tended to be.
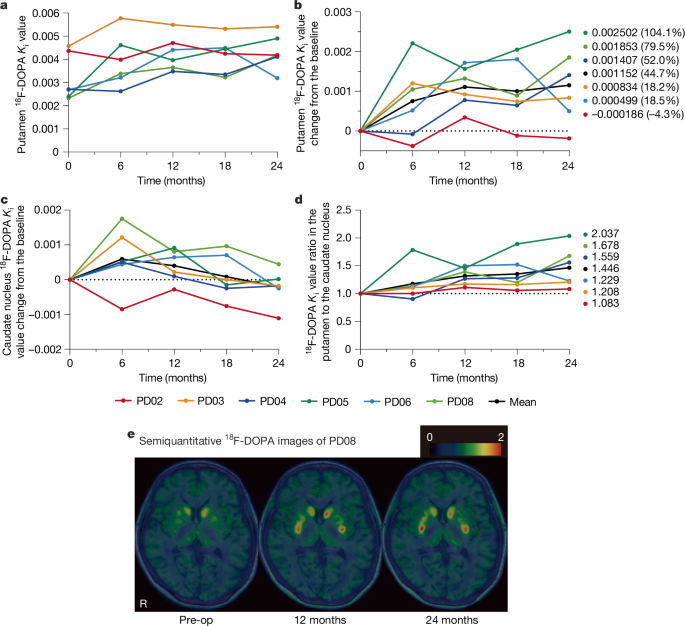
Symptoms of Parkinson’s disease: Measuring the effect of improving motor function:
Motor function tests were conducted after the effects of the Parkinson’s disease medication had worn off.
As a result of the tests, the values of four out of six patients improved after two years.
1. Some patients showed a significant improvement of 32 points.
2. Two out of the four patients saw their symptoms improve from “moderate” to “mild”.
3. One patient reported that their symptoms improved from “severe” to “moderate”.
On the other hand, the scores of two people worsened by several points. (= This is the same degree of deterioration as people who were receiving drug treatment for the same period.)
https://www3.nhk.or.jp/news/html/20250417/k10014781301000.html
Patients aged 50-69 years old: Clinical trials began in August 2018:
Cells made from iPS cells were transplanted into patients with Parkinson’s disease.
1. It was announced that the symptoms of four of the six people who were evaluated for effectiveness improved.
2. “Patients who cannot stand up on their own without taking medication” are sometimes able to stand and walk.
Published in the British scientific journal “Nature”:
Director Takahashi said, “Although the number of cases is small, we have been able to show solid results.”
It is expected that an application for manufacturing and sales approval will be submitted to the government based on the clinical trial data.
Director Takahashi said, “We hope to get approval by the end of the year.”
https://news.yahoo.co.jp/articles/412c9f9f26d0df079a9d084e90419a262c6755af?page=2