
COVID-19:レムデシビル第3相試験、限定的効果:ギリアドの株価急落(動画):
COVID-19:Gilead remdesivir has only modest benefit in large trial; shares fall:
COVID-19:吉利德remdesivir药物在大规模试验中仅具有适度的获益。股价下跌
2020年6月1日 23:55 JST
米ギリアド・サイエンシズ:
COVID-19中等症患者を対象に、抗ウイルス薬レムデシビルの大規模な臨床試験実施しました。
しかし、抗ウイルス薬レムデシビルの効果が、限定的なものにとどまったことが判明しました。
ギリアドの発表資料:
病状が中程度の患者グループを対象に、第3相試験を、実施しました。
- しかし、レムデシビル/5日間投与された患者は、標準的治療を受けた患者と比較し、わずかな改善しか見られません。
- 更に、10日間投与された別のグループでも、統計的な有意性が示されていません。
この結果を受け、「より長期間の投与で、なぜ効果が高まらないのかという疑問」を、引き起こしそうだ。
今回の臨床試験では、重症患者は対象になっていないとのこと。
Bloomberg
https://www.bloomberg.co.jp/news/articles/2020-06-01/QB90C9T1UM1301
Gilead drug has only modest benefit in large trial; shares fall
Gilead Sciences Inc.’s drug remdesivir showed only a limited benefit in a large trial,
a result that may shift perceptions of the first therapy cleared for use in severe cases of COVID-19.
In the phase 3 trial,
a group of moderately ill, hospitalized patients getting the drug for five days
showed a modest improvement compared to those getting the standard of care, the company said in a statement.
But another group getting the drug for 10 days didn’t show a statistically significant improvement, which is likely to raise questions about why a longer course doesn’t help more.
The shares were down 1.7 per cent in trading before the markets opened in New York, after earlier falling as much as 6.4 per cent. Severely ill patients weren’t included in the trial.
BNN Bloomberg
https://www.bnnbloomberg.ca/gilead-drug-has-only-modest-benefit-in-large-trial-shares-fall-1.1443895
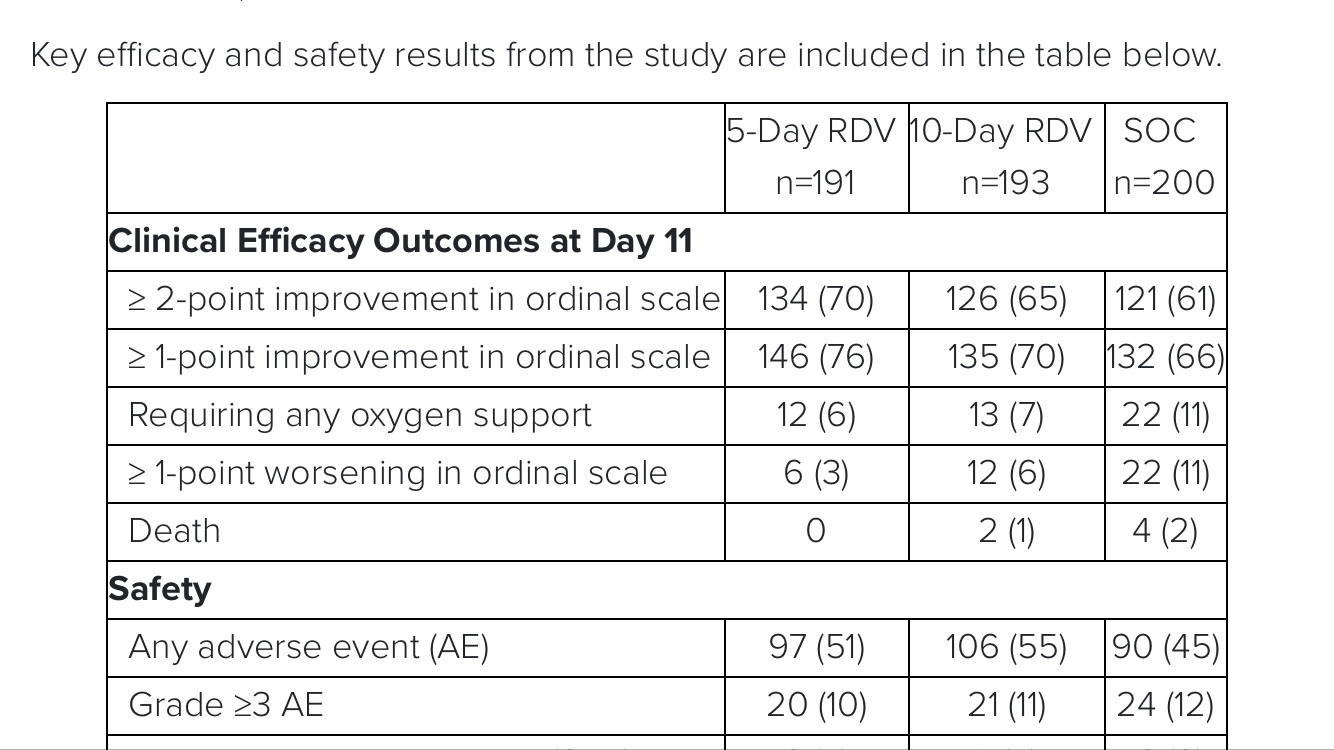
Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate COVID-19
FOSTER CITY, Calif.–(BUSINESS WIRE)– Gilead Sciences, Inc. (Nasdaq: GILD)
today announced topline results from the Phase 3 SIMPLE trial in hospitalized patients with moderate COVID-19 pneumonia.
This open-label study evaluated 5-day and 10-day courses of the investigational antiviral remdesivir plus standard of care, versus standard of care alone.
The study demonstrated that patients in the 5-day remdesivir treatment group were 65 percent more likely to have clinical improvement at Day 11 compared with those in the standard of care group (OR 1.65 [95% CI 1.09-2.48]; p=0.017).
The odds of improvement in clinical status with the 10-day treatment course of remdesivir versus standard of care were also favorable, trending toward but not reaching statistical significance (OR 1.31 [95% CI 0.88-1.95]; p=0.18).
No new safety signals
were identified with remdesivir across either treatment group.
Gilead plans to submit the full data for publication in a peer-reviewed journal in the coming weeks.
韓国、レムデシビルの輸入を承認:新型コロナ治療向け
[ソウル 3日 ロイター] –
韓国食品医薬品安全庁:
6月3日、新型コロナウイルス感染症治療薬として米ギリアド・サイエンシズの抗ウイルス薬「レムデシビル」を輸入することを承認しました。
政府の諮問委員会:
先週、「レムデシビルの試験で効果が認められた」と結論付け、保健当局が輸入を要請していた。
食品医薬品安全庁は、早期の輸入に向け、ギリアドや韓国疾病予防管理局(KCDC)など関係機関と連携する方針を示した。
ロイター
https://jp.reuters.com/article/health-coronavirus-southkorea-drug-idJPKBN23A0H8