
COVID-19:Gilead remdesivir has only modest benefit in large trial; shares fall
Gilead Sciences Inc.’s drug remdesivir showed only a limited benefit in a large trial,
a result that may shift perceptions of the first therapy cleared for use in severe cases of COVID-19.
In the phase 3 trial,
a group of moderately ill, hospitalized patients getting the drug for five days
showed a modest improvement compared to those getting the standard of care, the company said in a statement.
But another group getting the drug for 10 days didn’t show a statistically significant improvement, which is likely to raise questions about why a longer course doesn’t help more.
The shares were down 1.7 per cent in trading before the markets opened in New York, after earlier falling as much as 6.4 per cent. Severely ill patients weren’t included in the trial.
BNN Bloomberg
https://www.bnnbloomberg.ca/gilead-drug-has-only-modest-benefit-in-large-trial-shares-fall-1.1443895
Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate COVID-19
FOSTER CITY, Calif.–(BUSINESS WIRE)– Gilead Sciences, Inc. (Nasdaq: GILD)
today announced topline results from the Phase 3 SIMPLE trial in hospitalized patients with moderate COVID-19 pneumonia.
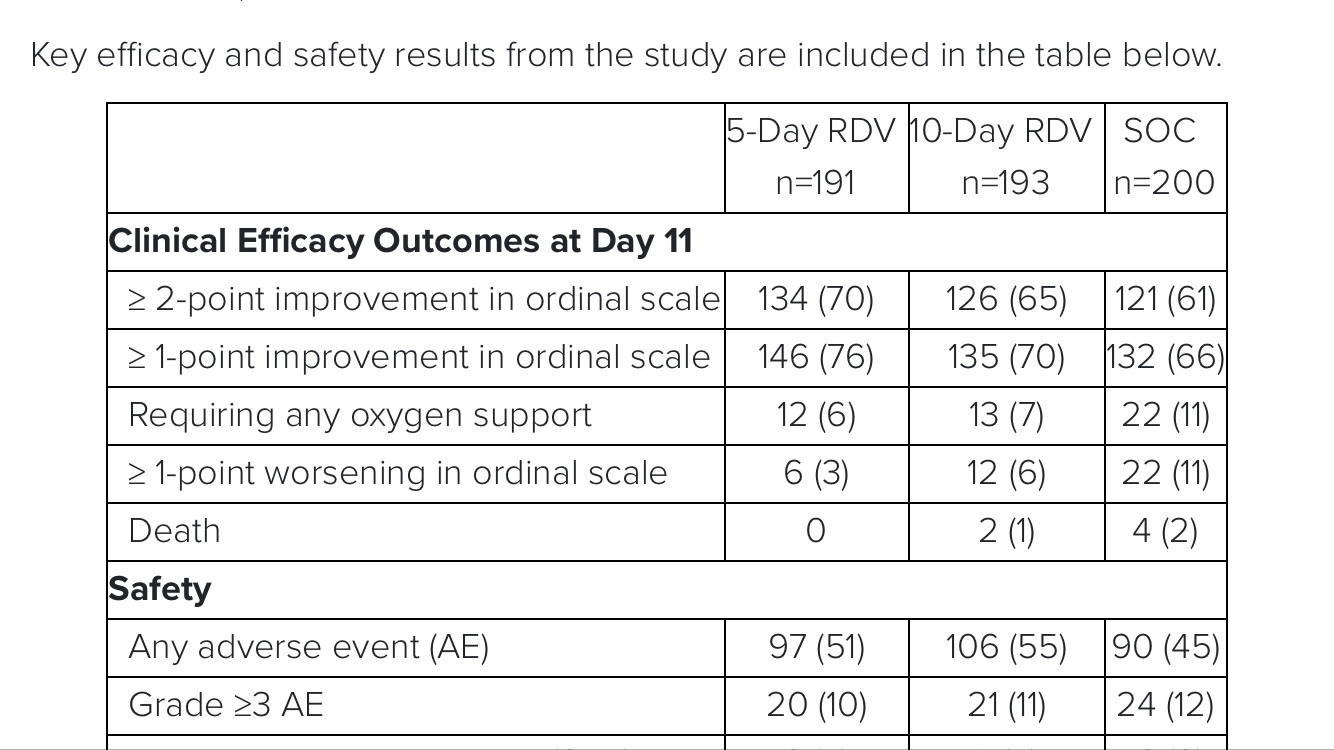
This open-label study evaluated 5-day and 10-day courses of the investigational antiviral remdesivir plus standard of care, versus standard of care alone.
The study demonstrated that patients in the 5-day remdesivir treatment group were 65 percent more likely to have clinical improvement at Day 11 compared with those in the standard of care group (OR 1.65 [95% CI 1.09-2.48]; p=0.017).
The odds of improvement in clinical status with the 10-day treatment course of remdesivir versus standard of care were also favorable, trending toward but not reaching statistical significance (OR 1.31 [95% CI 0.88-1.95]; p=0.18).
No new safety signals
were identified with remdesivir across either treatment group.
Gilead plans to submit the full data for publication in a peer-reviewed journal in the coming weeks.
South Korea Approves Import of Remdesibir: For New Corona Treatment
[Seoul 3rd Reuters]-
Korean Food and Drug Administration:
On June 3, we approved the import of Gilead Sciences’ antiviral drug “lemdecibir” as a new coronavirus infection treatment drug.
Government Advisory Board:
Last week, health officials called for imports, concluding that it was “effective in a trial with lemdecivir.”
The Food and Drug Administration has indicated that it will cooperate with related organizations such as Gilead and the Korea Disease Control and Control Office (KCDC) for early import.
Reuters
https://jp.reuters.com/article/health-coronavirus-southkorea-drug-idJPKBN23A0H8