

Kyoto University: Making an alloy that mixes 8 kinds of precious metals:
-Power far exceeding platinum catalyst-
Kyoto University
Shinshu UniversityThe research group succeeded for the first time in the world in “making an alloy that mixes all precious metals”.
“It is a power material that conventional alloys do not have.”
Hiroshi Kitagawa
Professor, Kyoto UniversityThe group made a liquid in which eight kinds of precious metal ions were dissolved,
It was gradually added in the form of drops to an organic solvent at 230 ° C.
Alloy ultrafine particles:
In a high-temperature solvent, “each ion returns to an atom at once, gathers, and mixes uniformly.”
By this method, we succeeded in producing alloy ultrafine particles.
8 kinds of precious metals:
Precious metals are rare and resistant to corrosion.
Eight types of gold, silver, platinum, palladium, rhodium, iridium, ruthenium, and osmium.
Use of alloy:
It is a catalyst that accelerates the reaction when applying voltage to water to generate hydrogen and oxygen.
Hydrogen is expected as a next-generation energy that does not emit CO2 when burned.
Research aimed at efficient reactions will proceed.
This alloy:
The reaction efficiency was more than 10 times that of platinum.
Mr. Kitagawa says, “The activity has improved dramatically.”
Asahi Shimbun Digital
https://www.asahi.com/articles/ASQ2H4TNMQ2GPLBJ005.html
Noble-Metal High-Entropy-Alloy Nanoparticles: Atomic-Level Insight into the Electronic Structure
Abstract
The compositional space of high-entropy-alloy nanoparticles (HEA NPs)
significantly expands the diversity of the materials library.
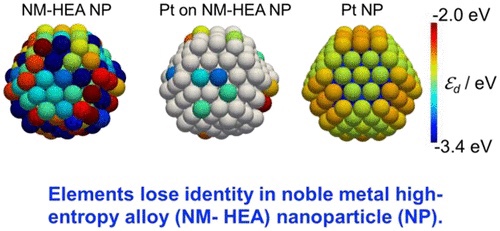
Every atom in HEA NPs
has a different elemental coordination environment, which requires knowledge of the local electronic structure at an atomic level.However,
such structure has not been disclosed experimentally or theoretically.We synthesized
HEA NPs composed of all eight noble-metal-group elements (NM-HEA) for the first time.Their electronic structure was revealed
by hard X-ray photoelectron spectroscopy and density function theory calculations with NP models.
The NM-HEA NPs
have a lower degeneracy in energy level compared with the monometallic NPs, which is a common feature of HEA NPs.The local density of states (LDOS) of every surface atom was first revealed.
Some atoms of the same constituent element in HEA NPs have different LDOS profiles, whereas atoms of other elements have similar LDOS profiles.
In other words,
one atom in HEA loses its elemental identity and it may be possible to create an ideal LDOS by adjusting the neighboring atoms.The tendency of the electronic structure change was shown by supervised learning.
The NM-HEA NPs showed 10.8-times higher intrinsic activity for hydrogen evolution reaction than commercial Pt/C, which is one of the best catalysts.
Journal of the American Chemical Society