Quantitative Research Organization: Hydrogen storage alloy, No rare metals used: Aluminum and iron
-Analyzing Al3FeH4 alloy with SPring-8-
October 11, 2021
Technology to carry hydrogen:
Attention is focused on hydrogen fuel with the aim of “decarbonization.”
Research and development of technology for efficiently transporting hydrogen is in progress.
Quantitative Research Organization (QST):
The research team succeeded in “development of an alloy that can efficiently occlude hydrogen without rare metals”.
If this inexpensive transportation method is established, it will lead to the expansion of hydrogen utilization.
Helps achieve the “government goal of reducing greenhouse gas emissions to virtually zero by 2050.”
Nihon Keizai Shimbun
https://www.nikkei.com/article/DGXZQOUC274740X20C21A9000000/
QST: No rare elements used: Aluminum and iron store hydrogen
-Leading new developments with the development of hydrogen storage alloys-
National Institutes for Quantum and Radiological Science and Technology (QST)
Research group
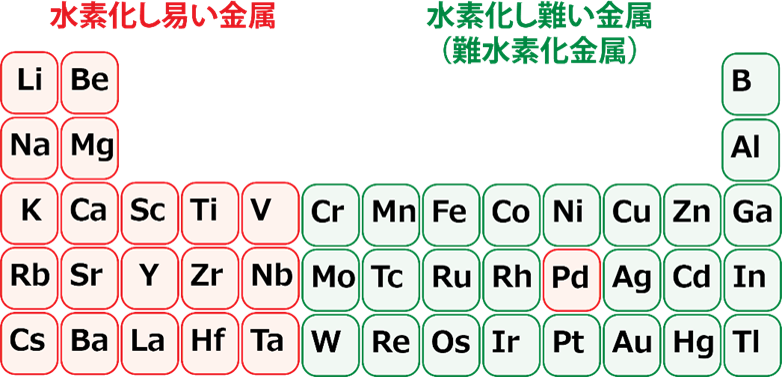
discovered that “a combination of aluminum and iron, which has abundant resources, can store hydrogen.”
Without containing rare elements as in the past
Can store hydrogen compactly,
The possibility of hydrogen storage alloy was shown.
Research results:
The research group repeated the following trial and error.
About alloys of aluminum and iron
Alloy composition, hydrogenation temperature, pressure, etc.
Elucidated the conditions for occluding hydrogen.
resulting in,
An alloy with a composition of Al13Fe4,
Under high pressure of 70,000 atm or more
React with high temperature hydrogen of 650 ℃ or higher,
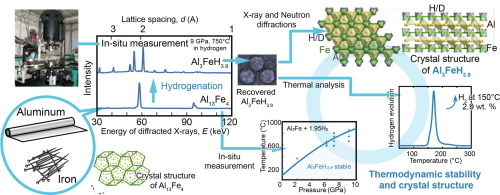
We synthesized a new metal hydride (alloy that occludes hydrogen) Al3FeH4.
High weight hydrogen density:
When the weight hydrogen density was calculated, it was “2.9% by weight”.
This amount is three times higher than for aluminum-copper alloys.
Current hydrogen storage system:
Currently, stationary hydrogen storage systems are in use.
A typical hydrogen storage alloy using rare metals
LaNi5 “1.4% by weight” and
Compared to TiFe “1.9% by weight” etc.
It turned out to be at the same level.
Analysis at SPring-8,
a large synchrotron radiation facility:
In “Hydrogenation condition survey of aluminum and iron alloy”,
We used the high voltage device installed in the beamline BL14B1 dedicated to SPring-8 / QST.
National Institutes for Quantum and Radiological Science and Technology
https://www.qst.go.jp/site/press/20210729.html
Hydrogen storage by earth-abundant metals, synthesis and characterization of Al3FeH3.9
Highlights
• A novel hydrogen storage material, Al3FeH3.9, is synthesized from the earth-abundant metals aluminum and iron.
• Al3FeH3.9 contains only metals with low hydrogen affinity, whereas other reported hydrides contain metals with high hydrogen affinity.
• The crystal structure of the synthesized Al3FeD3.9 suggests that the mixing of the chemical state of hydrogen stabilizes Al3FeH3.9.
• The hydrogen content of Al3FeH3.9 (2.9 wt%) exceeds that of conventional LaNi5Hx (1.4 wt%) and TiFeHx (1.9 wt%).-
ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0264127521005074
Synchrotron Radiation Research | High Pressure Science and Stress Research Group