Austria: Producing early fetal cells from fertilized eggs: Recreating implantation
-Creating a’blastocyst-like cell mass’with iPS cells-
Austria
Research group
Presented by an Austrian research group.
Using human iPS cells, etc.
In the early stages when a fertilized egg becomes a foetation,
We made a mass of expressed cells.
The cell mass succeeded in “recreating the appearance of implantation in an artificially created tissue similar to the uterus.”
Austrian Academy of Sciences
Institute for Molecular Biological Engineering
Researcher Harunobu Kagawa
Nicholas Libron
Published in the scientific journal “Nature”.
Human iPS cells and ES cells:
The research group cultivates human iPS cells and ES cells by adding substances that promote cell differentiation.
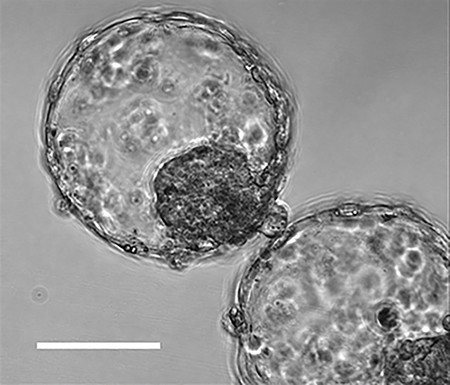
Creating a “blastocyst”:
In the early stage, about 5 days after fertilization, a mass of cells like a “blastocyst” was formed.
Moreover,
The cell mass “adhered to an artificially created tissue that resembled the endometrium of a human uterus and was able to reproduce the initial state of implantation.”
Bioethics Guidelines:
The research group completed the culture from fertilization to the 13th day according to the bioethics guidelines.
“It is a result that will lead to elucidation of the cause of infertility and research on treatment.”
Medical | NHK News
https://www3.nhk.or.jp/news/html/20211212/k10013384951000.html
Scientists recreate blastocysts with iPS, ES cells
A group of scientists
have successfully created human blastocyst models from induced pluripotent stem cells and embryonic stem cells.They say
they recreated the early stages of implantation, and the research could lead to further understanding of infertility and its treatment.The research was conducted by a number of researchers including a group
at the Institute of Molecular Biotechnology of the Austrian Academy of Science.
The scientists say
they observed implantation of the cell models called blastoids into a recreated uterine wall that was cultured from human uterine cells.British science magazine Nature carried the article online.
The scientists
cultured iPS cells and embryonic stem cells to create blastoids which are similar to blastocysts of five to seven days after fertilization.The blastoids are similar to natural blastocysts in size and in genetic characteristics of that stage.
The scientists believe their functions are similar.
They stopped culturing after 13 days of fertilization following international ethics guidelines on life science.
Researcher Kagawa Harunobu of the Institute of Molecular Biotechnology of the Austrian Academy of Science
said the results indicate an increase in the success rate of IVF and open up opportunities for people to plan pregnancies more precisely.
NHK WORLD-JAPAN News
https://www3.nhk.or.jp/nhkworld/en/news/20211212_09/
Human blastoids model blastocyst development and implantation
Abstract One week after fertilization, human embryos implant into the uterus.
This event requires that
the embryo forms a blastocyst consisting of a sphere encircling a cavity lodging the embryo proper.Stem cells can form a blastocyst model, which we termed blastoid1.
Here we show that
naive human pluripotent stem cells (PXGL hPSCs)2 triply inhibited for the Hippo, TGF-β, and ERK pathways efficiently (>70%) form blastoids generating blastocyst-stage analogs of the 3 founding lineages (>97% trophectoderm, epiblast, and primitive endoderm)according to the sequence and timing of blastocyst development.
Blastoids spontaneously form the first axis and we observe that
the epiblast induces the maturation of the polar trophectoderm that consequently acquires the specific capacity to attach to hormonally-stimulated endometrial cells, as during implantation.
Such a human blastoid is a faithful, scalable, and ethical model to explore human implantation and development3,4.
Nature