奥地利:从受精卵生产早期胎儿细胞:重新植入
-用 iPS 细胞制造“囊胚样细胞团”-
奥地利
研究组
由奥地利研究小组提出。
使用人iPS细胞等
在受精卵发育的早期阶段,
我制作了大量表达细胞。
细胞团成功地“在类似于子宫的人造组织中重建了植入的外观”。
奥地利科学院
分子生物工程研究所
研究员香川春信
尼古拉斯·利布龙
发表在科学期刊《自然》上。
人 iPS 细胞和 ES 细胞:
该研究小组通过添加促进细胞分化的物质来培养人类iPS细胞和ES细胞。
创建“囊胚”:
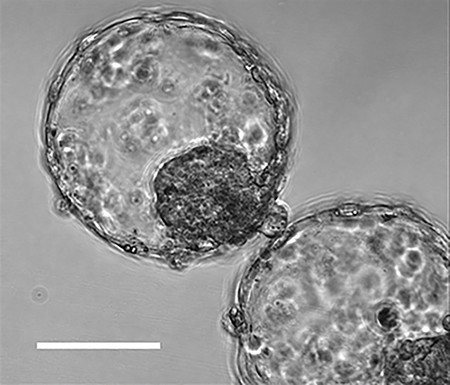
在早期,受精后大约5天,形成了像“囊胚”一样的细胞团。
而且,
细胞团“附着在类似于人类子宫内膜的人造组织上,能够重现植入的初始状态。”
生物伦理准则:
课题组按照生物伦理学指南完成了从受精到第13天的培养。
“这一结果将导致阐明不孕症的原因和治疗研究。”
医疗 | NHK 新闻
https://www3.nhk.or.jp/news/html/20211212/k10013384951000.html
Scientists recreate blastocysts with iPS, ES cells
A group of scientists
have successfully created human blastocyst models from induced pluripotent stem cells and embryonic stem cells.They say
they recreated the early stages of implantation, and the research could lead to further understanding of infertility and its treatment.The research was conducted by a number of researchers including a group
at the Institute of Molecular Biotechnology of the Austrian Academy of Science.
The scientists say
they observed implantation of the cell models called blastoids into a recreated uterine wall that was cultured from human uterine cells.British science magazine Nature carried the article online.
The scientists
cultured iPS cells and embryonic stem cells to create blastoids which are similar to blastocysts of five to seven days after fertilization.The blastoids are similar to natural blastocysts in size and in genetic characteristics of that stage.
The scientists believe their functions are similar.
They stopped culturing after 13 days of fertilization following international ethics guidelines on life science.
Researcher Kagawa Harunobu of the Institute of Molecular Biotechnology of the Austrian Academy of Science
said the results indicate an increase in the success rate of IVF and open up opportunities for people to plan pregnancies more precisely.
NHK WORLD-JAPAN News
https://www3.nhk.or.jp/nhkworld/en/news/20211212_09/
Human blastoids model blastocyst development and implantation
Abstract One week after fertilization, human embryos implant into the uterus.
This event requires that
the embryo forms a blastocyst consisting of a sphere encircling a cavity lodging the embryo proper.Stem cells can form a blastocyst model, which we termed blastoid1.
Here we show that
naive human pluripotent stem cells (PXGL hPSCs)2 triply inhibited for the Hippo, TGF-β, and ERK pathways efficiently (>70%) form blastoids generating blastocyst-stage analogs of the 3 founding lineages (>97% trophectoderm, epiblast, and primitive endoderm)according to the sequence and timing of blastocyst development.
Blastoids spontaneously form the first axis and we observe that
the epiblast induces the maturation of the polar trophectoderm that consequently acquires the specific capacity to attach to hormonally-stimulated endometrial cells, as during implantation.
Such a human blastoid is a faithful, scalable, and ethical model to explore human implantation and development3,4.
Nature