Kyoto University: Making placental cells from iPS: Human naive iPS cells
-Expectations for elucidation of pregnancy complications-
Lecturer Yasuhiro Takashima, Kyoto University:
Placental cells were generated from human induced pluripotent stem cells (iPS cells) in an experimental vessel.
Lecturer Yasuhiro Takashima of Kyoto University announced on April 7th in the electronic version of the American scientific journal Cell Stem Cell.
Placental cell formation in the laboratory vessel:
The placenta supplies oxygen and nutrients to the foetation in the womb.
If there is an abnormality in the formation of placental cells
Infertility and fetal dysgenesis,
Maternal hypertension, etc.
Helps clarify pregnancy complications.
“It will be a model for the process of placental cell formation and will help clarify the cause,” said Takashima.
Use of iPS cells (naive type):
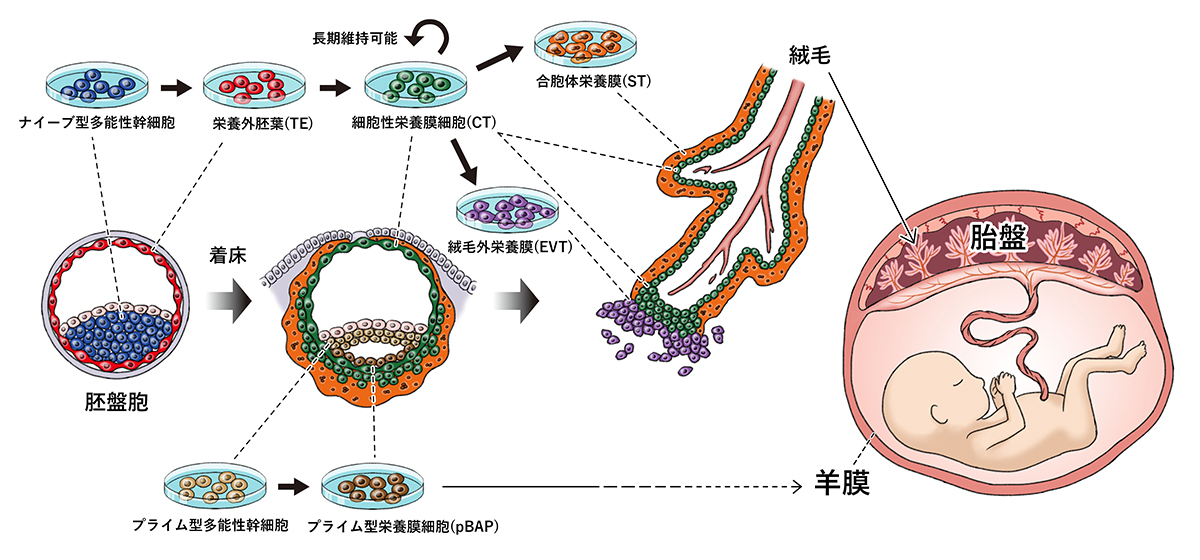
Lecturer Takashima et al. Used “iPS cells (naive type) improved to be closer to fertilized eggs”.
It is not an iPS cell (prime type) originally developed by Professor Shinya Yamanaka of Kyoto University.
The substances and medium to be added to the culture solution were devised.
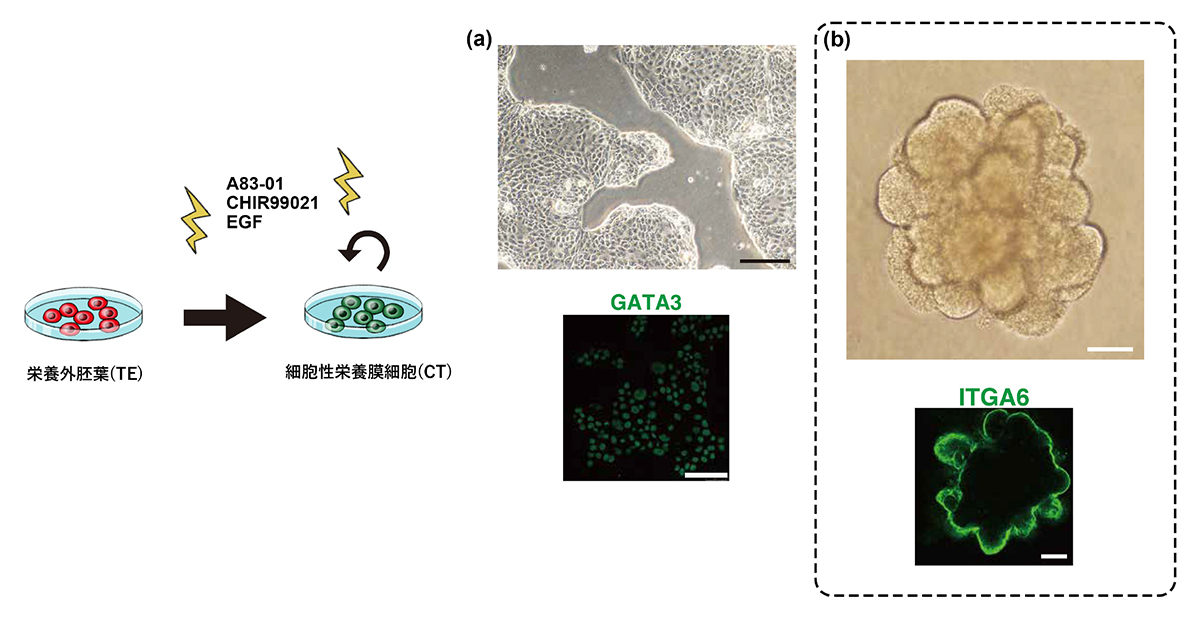
As a result, “the process of forming three types of placental cells from vegetative ectoderm” was reproduced.
Kyoto University: Jiji.com
https://www.jiji.com/jc/article?k=2021040800024&g=soc
Make placental cells from human naive iPS cells
~ Succeeded in constructing a placenta development model outside the body ~
point:
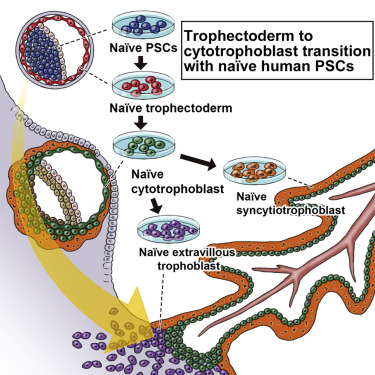
For the first time, we succeeded in producing vegetative ectoderm (TE) from human naive iPS cells, and confirmed the differentiation into a cell group that continues to placental cells.
In the study of early human embryos, which was technically and ethically difficult, we were able to construct a model that mimics the differentiation process of placental cells in vitro.
It is possible to investigate changes in placental cells before and after implantation.
We have clarified a part of the early differentiation lineage of naive iPS cells, which are expected as next-generation iPS cells.
CiRA | Center for iPS Cell Research and Application, Kyoto University
https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/210408-000001.html
Stem cells reproduce the beginnings of the placenta
The Yasuhiro Takashima lab shows that naïve iPS cells can be induced to form all the stages that mimic early placenta development in humans.
Gynecologist Shingo Io knows that
during birth there will not only be a baby leaving the mother’s body.
Joining the cries that bring joy to the room will be a silent entity, the placenta.
Like the baby, this tissue only began to grow upon conception, but little is known about how the placenta develops inside the mother.
A new study by Io, CiRA Junior Associate Professor Yasuhiro Takashima and colleagues reports how iPS cells can be used to study this development.
The study is the first to show that
iPS cells have this potential and will be invaluable for understanding the many factors that lead to miscarriage.
No matter how old or how big, all of us began as a fertilized egg, which is nothing more than a single cell.
This one cell will grow into an embryo, which eventually leads to a fully formed human body.
That one cell also grows into extra-embryonic tissue that becomes the placenta.
While not part of the baby’s body, the placenta is essential for healthy growth.
Historically tossed aside like a bloody towel, it is now studied intensively.
CiRA | Center for iPS Cell Research and Application, Kyoto University
https://www.cira.kyoto-u.ac.jp/e/pressrelease/news/210408-000001.html
Capturing human trophoblast development with naive pluripotent stem cells in vitro
Highlights
• Trophectoderm can be generated from naive human pluripotent stem cells
• Human trophoblast stem cells represent post-implantation cytotrophoblast
• Trajectory of trophoblast from pre- to post-implantation is drawn by our model
• ACE is essential for specification and maintenance of cytotrophoblast stem cells
ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S1934590921001193