Kyoto University: Utilizing iPS cells for cancer immunotherapy: Proliferating killer T cells
-Cost of cancer immunotherapy less than 1/10-
Cancer immunotherapy:
Killer T cells that attack cancer cells in the body,
“Immunotherapy” transplanted from patients and donors
It is valid.
However, high costs have traditionally been an issue.
Center for iPS Cell Research and Application, Kyoto University
A group of new professors Kaneko
We aimed to proliferate T cells using iPS cells.
However, until now, “aggression to cancer cells in the process of proliferation” has decreased.
A substance called IL21:
Therefore, a substance called IL21 was added to proliferate T cells.
“With IL21, we succeeded in maintaining aggression.”
Mass production of T cells:
Professor Kaneko
If mass production of T cells becomes possible, it will cost less than one tenth of the conventional cost.
He says he can get immunotherapy.
https://www.fnn.jp/articles/-/253309
Produced “Killer T cell clone with high proliferative potential”
-Expected to be applied to adopted immunotherapy-
wrap up:
A treatment method (adoptive immunotherapy) in which immune cells are cultured and activated in vitro and transplanted is attracting attention.
However, in the process of stimulating and proliferating T cells, cell exhaustion and aging have become issues.
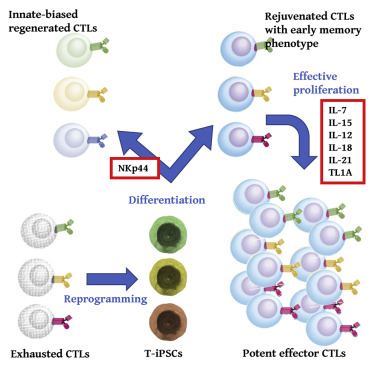
Succeeded in “cloning killer T cells with higher proliferation” using iPS cell technology
Adoptive immunotherapy:
It is expected to be a powerful means against chronic infectious diseases such as cancer and HIV / AIDS.
However,
At the stage of producing a large amount of T cells,
Due to cell exhaustion, aging, and differentiation
The challenge is to reduce the ability to grow.
Improved differentiation induction method
Therefore, by improving the differentiation induction method,
Has high self-renewal ability,
Using powerful effector T cells,
We created iPSC-CTL, which is characteristic of early memory T cells. Twice
Proliferation culture under optimal conditions:
iPSC-CTL proliferated more than 1015 times when grown and cultured under optimized conditions.
Using the iPSC-CTL produced by this method,
By redifferentiating and expanding culture
Whether autologous or allogeneic
It is expected that “memory T cells and effector T cells can be supplied”.
This research result:
It was published in “Molecular Therapy” on October 6, 2021.
News | CiRA | Center for iPS Cell Research and Application, Kyoto University
https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/211013-160000.html
Generation of highly proliferative, rejuvenated cytotoxic T cell clones through pluripotency reprogramming for adoptive immunotherapy
Adoptive immunotherapy
has emerged as a powerful approach to cure cancer and chronic infections.
Currently, the generation of a massive number of T cells that provide long-lasting immunity
is challenged by exhaustion and differentiation-associated senescence, which inevitably arise during in vitro cloning and expansion.
To circumvent these problems,
several studies have proposed an induced pluripotent stem cell (iPSC)-mediated rejuvenation strategy to revitalize the exhausted/senescent T cell clones.
Because iPSC-derived cytotoxic T lymphocytes (iPSC-CTLs) generated via commonly
used monolayer systems have unfavorable, innate-like features such as aberrant natural killer (NK) activity and limited replication potential,
we modified the redifferentiation culture to generate CD8αβ+CD5+CCR7+CD45RA+CD56−-adaptive iPSC-CTLs.
The modified iPSC-CTLs
exhibited early memory phenotype, including high replicative capacity and the ability to give rise to potent effector cells.
In expansion culture with an optimized cytokine cocktail,
iPSC-CTLs proliferated more than 1015-fold in a feeder-free condition.
Our redifferentiation and expansion package of early memory iPSC-CTLs
could supply memory and effector T cells for both autologous and allogeneic immunotherapies.Molecular Therapy