Kyushu University, RIKEN: Genes that turn cells into eggs: iPS application for infertility treatment
Kyushu University, RIKEN:
Genes that make cells “eggs”:
We have identified the genes required for the formation of the special cytoplasm (egg cytoplasm) contained in the egg.
Mass production in a short period of time:
Embryonic stem cells (ES cells)
From induced pluripotent stem cells (iPS cells)
We succeeded in producing a large amount of cells with egg cytoplasm (egg-like cells) in a short period of time.
The treatise was published in the English scientific journal Nature by the 20th.
Current affairs dot com
https://www.jiji.com/jc/article?k=2021012000257&g=soc
Identify the genes that make up the egg
Kyushu University Graduate School of Medicine: Professor Hayashi, Assistant Professor Hamasaki (University of Washington),
RIKEN: Kitajima Team Leader
reaserch result:
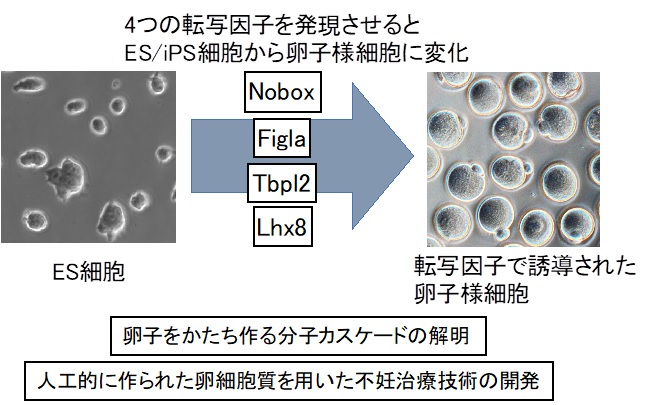
Identified a group of genes that form mouse eggs.
This gene group was introduced into embryonic stem (ES) cells and induced pluripotent stem (iPS) cells.
In a short period of time, we succeeded in producing a large number of egg-like cells.
Eight genes (transcription factors):
We carefully investigated the process of making an egg.
As a result, we identified eight genes (transcription factors) required for the formation of egg cytoplasm.
Surprisingly:
When the eight genes are expressed in ES cells and iPS cells,
The cytoplasm grows rapidly,
It changed into an egg-like cell with fertilizing ability.
Mass production in a short period of time:
It is possible to produce a large amount of “biologically and medically valuable egg cytoplasm” in a shorter period of time than before.
Due to this achievement:
“Elucidation of the formation mechanism of egg cytoplasm necessary for individual development” and
“Development of infertility treatment technology using artificially made egg cytoplasm” is expected.
Results of this research:
It was published in the international academic journal “Nature” on December 16, 2020 (Wednesday) at 16:00 (UK standard time).
Kyushu University
https://www.kyushu-u.ac.jp/ja/researches/view/538
Nature volume 589, pages264–269(2021)
Cite this article 15k Accesses 1 Citations 543 Altmetric Metrics details
Reconstitution of the oocyte transcriptional network with transcription factors
Abstract
During female germline development, oocytes
become a highly specialized cell type and form a maternal cytoplasmic store of crucial factors.
Oocyte growth
is triggered at the transition from primordial to primary follicle and is accompanied by dynamic changes in gene expression1, but the gene regulatory network that controls oocyte growth remains unknown.
Here we identify a set of transcription factors that are sufficient to trigger oocyte growth.
By investigation of the changes in gene expression and functional screening using an in vitro mouse oocyte development system,
we identified eight transcription factors, each of which was essential for the transition from primordial to primary follicle.
Notably, enforced expression of these transcription factors
swiftly converted pluripotent stem cells into oocyte-like cells that were competent for fertilization and subsequent cleavage.
These transcription-factor-induced oocyte-like cells were formed without
specification of primordial germ cells, epigenetic reprogramming or meiosis,
and demonstrate that oocyte growth and lineage-specific de novo DNA methylation are separable from the preceding epigenetic reprogramming in primordial germ cells.
This study
identifies a core set of transcription factors for orchestrating oocyte growth, and provides an alternative source of ooplasm, which is a unique material for reproductive biology and medicine.
Nature