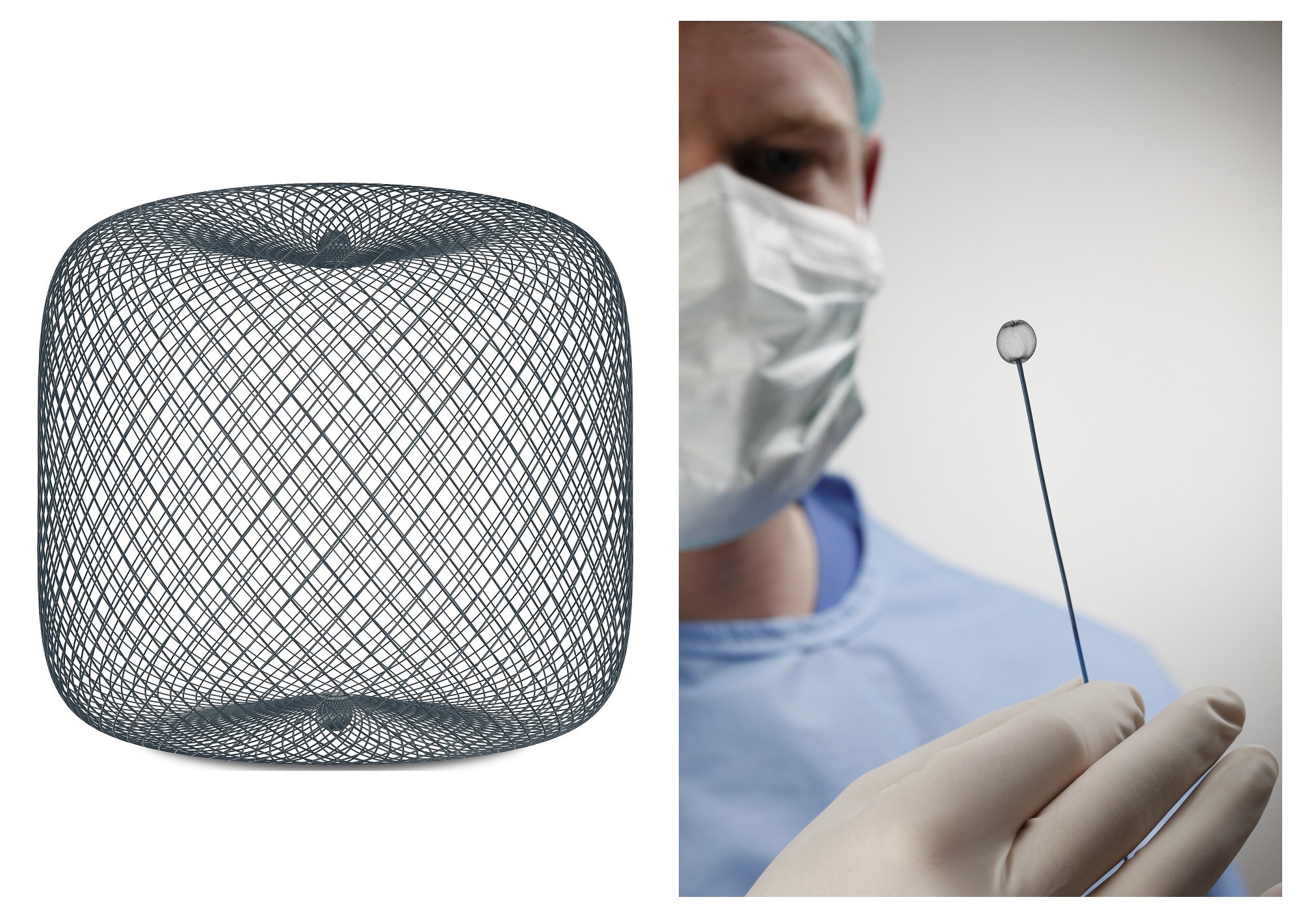
FDA approves Terumo’s embolic device “WEB” – for treatment of cerebral aneurysm
Terumo Corporation
A bag-shaped embolic device “WEB” used for cerebral aneurysm treatment acquired market approval in the United States.
WEB is the first product in the US as a bag-shaped embolic device.
As a new treatment for cerebral aneurysms, we plan to start sales in FY 2019.
In WEB, the shape memory alloy made of nickel titanium has become a fine mesh bag shape,
It is a mechanism to let the catheter reach the affected part of the brain, spread it in the Cobb, block the flow of blood.
Clinical trials conducted in the United States
Their efficacy and safety were evaluated, and cases where cobs were found in the vascular bifurcation area, which is considered difficult to treat with conventional coils and the like, was regarded as the scope of application.
It has been released in Europe in 2010 and has over 6000 cases of use worldwide.
Press Release | Terumo