
COVID-19: Toray Blood Purifier TORAYMYXIN®: Approved by Health Canada
April 21, 2020
Toray Industries, Inc .:
Toray has obtained “COVID-19 Treatment / Provisional Use Permit” for TORAYMYXIN® from Canada from Health Canada.
TORAYMYXIN® (Approval number: 20500BZZ00926000): Adsorption-type blood purification purifier for endotoxin removal
COVID-19:
With the increase in severity of SARS-CoV-2, respiratory failure and other organ failure develop.
Health Canada:
“Interim order respecting the Importation and sale of medical devices for use in relation to COVID-19” was established.
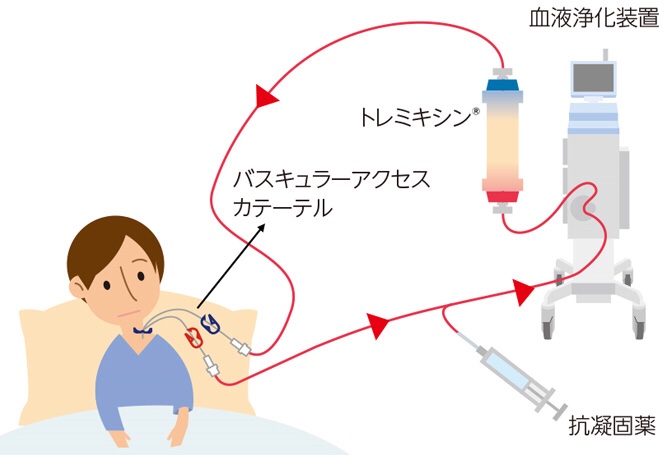
TORAYMYXIN®:
In 2003, we obtained an import permit for the treatment of septic shock.
Toray filed an application under the Interim order system and was approved for expanded use for the following indications on April 17th.
Temporary expansion:
Use toremixin in patients with COVID-19 who:
Acute respiratory failure without heart failure or fluid overload
Find diffuse alveolar damage
Pulmonary oxygenation capacity (PaO2 / FiO2) is less than 300mmHg
US FDA: Approved
In the United States, a licensed company obtained FDA approval for use in COVID-19 patients as a clinical trial in April.
https://www.toray.co.jp/news/life/detail.html?key=A3E6AB80420B5862492585510016C585
Apr. 22, 2020
Toray Industries, Inc.
Regarding an approval for the interim order of“
TORAYMYXIN®”
to treat COVID-19 patients in Canada
Tokyo, Japan, April 22, 2020 –
Toray Industries, Inc. and Toray Medical Co., Ltd. are pleased to announce that
Canadian Authority, Health Canada has issued an Interim Order for use of an endotoxin adsorption cartridge,
TORAYMYXIN®
to treat patients with the novel coronavirus (“COVID-19”). COVID-19,
the novel coronavirus (SARS-CoV-2) is an infectious disease that causes organ dysfunction including respiratory failure with worsening disease conditions.
As the COVID-19 pandemic
accelerates,“Interim order respecting the Importation and sale of medical devices for use in relation to COVID-19” has been established by Health Canada.
Toray has possessed the Canadian Import License for TORAYMYXIN® to treat patients with septic shock since its approval in 2003 (License Number: 63404).
In addition to the current indications,
Toray applied for the authorization of the interim order for TORAYMYXIN® to treat COVID-19 patients, and was granted expanded indications for use on April 17 as follows.
https://www.toray.com/news/life/detail.html?key=89229825E2E9D466492585520005F978