
COVID-19:Toray血液净化器Tremixin®:加拿大卫生部批准
2020年4月21日
东丽工业株式会社:
Toray已从加拿大卫生部获得了加拿大Tremixin®的“ COVID-19治疗/临时使用许可证”。
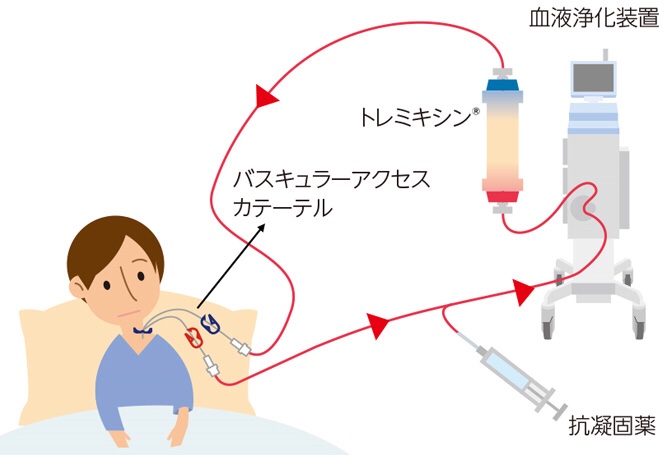
Tremyxin®(批准号:20500BZZ00926000):吸附型血液净化净化器,用于去除内毒素
COVID-19:
随着SARS-CoV-2严重程度的提高,出现呼吸衰竭和其他器官衰竭。
加拿大卫生部:
建立了“关于进口和销售与COVID-19有关的医疗设备的临时命令”。
Tremixin:
2003年,我们获得了治疗败血性休克的进口许可证。
东丽根据临时订单系统提交了申请,并于4月17日被批准扩展用于以下指示。
临时扩展:
在患有COVID-19的患者中使用toremixin:
急性呼吸衰竭,无心力衰竭或液体过多
查找弥漫性肺泡损伤
肺氧合能力(PaO2 / FiO2)小于300mmHg
美国FDA:批准
在美国,一家有执照的公司于4月份获得了FDA批准用于COVID-19患者的临床试验。
https://www.toray.co.jp/news/life/detail.html?key=A3E6AB80420B5862492585510016C585
https://www.toray.co.jp/news/life/detail.html?key=A3E6AB80420B5862492585510016C585
Apr. 22, 2020
Toray Industries, Inc.
Regarding an approval for the interim order of“
TORAYMYXIN®”
to treat COVID-19 patients in Canada
Tokyo, Japan, April 22, 2020 –
Toray Industries, Inc. and Toray Medical Co., Ltd. are pleased to announce that
Canadian Authority, Health Canada has issued an Interim Order for use of an endotoxin adsorption cartridge,
TORAYMYXIN®
to treat patients with the novel coronavirus (“COVID-19”). COVID-19,
the novel coronavirus (SARS-CoV-2) is an infectious disease that causes organ dysfunction including respiratory failure with worsening disease conditions.
As the COVID-19 pandemic
accelerates,“Interim order respecting the Importation and sale of medical devices for use in relation to COVID-19” has been established by Health Canada.
Toray has possessed the Canadian Import License for TORAYMYXIN® to treat patients with septic shock since its approval in 2003 (License Number: 63404).
In addition to the current indications,
Toray applied for the authorization of the interim order for TORAYMYXIN® to treat COVID-19 patients, and was granted expanded indications for use on April 17 as follows.
https://www.toray.com/news/life/detail.html?key=89229825E2E9D466492585520005F978