
Sumitomo Heavy Industries: World’s first accelerator / BNCT treatment system approval: for head and neck cancer
February 19, 2020
Sumitomo Heavy Industries: World’s first accelerator / BNCT treatment system
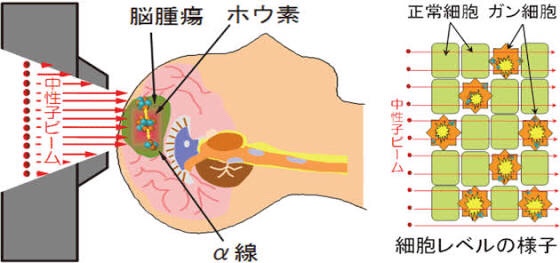
Sumitomo Heavy Industries, Ltd. has been conducting a Phase III clinical trial for head and neck cancer in collaboration with Stella Pharma on the “BNCT (Boron Neutron Capture Therapy) treatment system using an accelerator”.
Based on these results, we have applied for approval for the manufacture and sale of the accelerator / BNCT treatment system.
Accelerator / BNCT treatment system: Manufacturing and marketing approved
We are pleased to announce that the BNCT treatment system (NeuCure ™) and the BNCT dose calculation program (NeuCure ™ dose engine) have been approved.
Held on February 19, was deliberated in the Medical Devices and In Vitro Diagnostics Subcommittee of the Ministry of Health, Labor and Welfare Pharmaceutical Affairs and Food Sanitation Council.
In the future, if it is officially approved, it will be the world’s first medical device for BNCT using an accelerator.
2019 | Sumitomo Heavy Industries, Ltd.
https://www.shi.co.jp/info/2019/6kgpsq000000bvfu.html
Approval of BNCT treatment system using accelerator and medical device and in-vitro examination of BNCT dose calculation program
https://www.shi.co.jp/info/2019/6kgpsq000000bvfu-att/6kgpsq000000bvgg.pdf