Chiba Univ: Head and neck cancer treatment with iPS cells: NKT immune cells are administered
Professor Shinichiro Motohashi of Chiba University
Started a doctor-led clinical trial called “Immune cells made from iPS cells for cancer treatment”.
Head cancer / iPS treatment:
For 4 to 18 patients with cervical cancer that develops in the nose and mouth.
This is the first cancer treatment in Japan that uses iPS cells.
RIKEN:
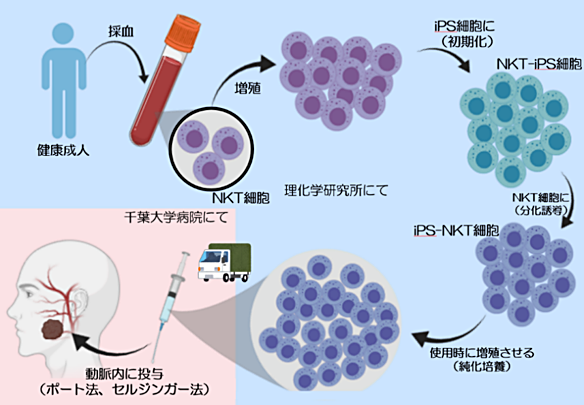
In clinical trials, iPS cells will be administered.
Immune cells and NKT cells are extracted from the blood of a healthy person.
Make iPS cells at RIKEN.
Chiba University Hospital:
Increase iPS cells in large quantities and grow them into NKT cells,
Administered 3 times every 2 weeks to the blood vessels leading to the affected area of the cancer patient.
On October 14, the first dose was started in the first patient.
If the problem does not occur, increase the dose and investigate the safety and efficacy.
How to use immune cells:
It has already been effective against blood cancer and was put into practical use in 2017.
This method is considered to be the fourth treatment method after surgery, radiation, and drug treatment.
Conventionally, the patient’s own immune cells were used,
This time, using iPS cells and embryonic stem cells (ES cells),
It is possible to produce high quality cells in large quantities.
It is expected to lead to cost reductions in cancer treatment.
Nihon Keizai Shimbun
https://www.nikkei.com/article/DGXMZO65341120S0A021C2I00000/
World’s first direct administration of iPS-NKT cells into blood vessels
June 25, 2020
Chiba University Hospital
Developed a treatment method using immune cells “NKT cells” as a new treatment method for head and neck cancer.
Produce iPS-NKT cells
Aim to further improve the survival rate in collaboration with RIKEN.
Using “iPS-NKT cells” that produced NKT cells from iPS cells,
The treatment to be administered to humans will be conducted as a doctor-initiated clinical trial. ..
Conducting a doctor-led clinical trial
Until now, “iPS-NKT cells” have never been directly administered into human blood vessels.
In this clinical trial, tolerability (evaluating the occurrence of side effects and examining the appropriate dose), safety, and efficacy will be confirmed.
For patients with head and neck cancer
It is a great hope for patients who are fighting head and neck cancer (cancer of the nose, mouth, throat, upper jaw, lower jaw, ears, etc.).
RIKEN
https://www.riken.jp/pr/news/2020/20200629_2/index.html
World’s first direct administration of iPS-NKT cells into blood vessels Started clinical trial with immuno-cell therapy for head and neck cancer
https://www.m.chiba-u.ac.jp/dept/jibika/files/4915/9348/3776/news_release_20200629.pdf