Tokyo Institute of Technology: High Li conductivity even at -20 ° C: Developed organic molecular crystals
-Development of organic molecular crystals with high Li conductivity-
Shizuoka University and Tokyo Institute of Technology:
Research group:
In November 2020, it announced that it has developed an “organic molecular crystal” that exhibits high lithium-ion conductivity even in low-temperature environments.
The conductivity at -20 ° C is almost 100 times higher than before, and it can be installed in automobiles with cold climate specifications.
It is applied as a solid electrolyte for all-solid-state batteries.
Benefits of all-solid-state batteries:
Compared to current lithium-ion batteries
It can suppress liquid leakage and ignition, and
This is because it can improve safety.
The series-stacked structure makes it possible to reduce the size and weight of storage batteries.
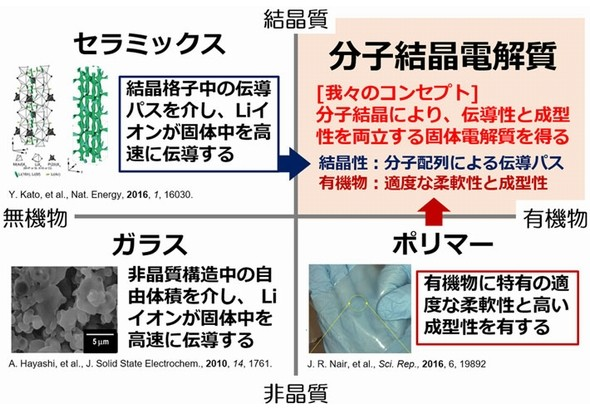
Conventional solid electrolyte:
So far, “ceramics”, “glass” and polymer materials have been studied.
However, there are various difficulties in the practical application and mass production of all-solid-state batteries.
This solid electrolyte:
As a new solid electrolyte, we focused on “molecular crystals” (crystalline organic substances) in which molecules are regularly arranged in a crystal lattice.
Combination of lithium salts and organic molecules,
With proper selection of reaction ratio,
It is possible to construct an ionic conduction path consisting of molecules,
Moreover, the molecular crystals are reasonably flexible.
A good interface can be formed between the electrolyte and the electrode of the all-solid-state battery.
Material design of molecular crystals:
So far, the research group has been involved in the material design of molecular crystals with high Li-ion conductivity.
“Reduction of interaction around Li”
“Reducing the distance between Li and Li”,
“Existence of vacant coordination”,
Has been shown to be important.
This experiment:
Component selection:
Lithium amide (LiFSA: Li {N (SO2F) 2})
Succinonitrile (SN: NCCH2CH2CN)
The above two materials were used.
Mixing and heating process:
LiFSA and SN,
In an argon atmosphere
Mix at a molar ratio of 1: 2 and
It was heated until a uniform melt was obtained.
Results of mixing and heating:
The melt obtained by heating Li (FSA) (SN) 2 is
When cooled to below the melting point of 59.5 ° C,
It is crystallized again and the three-dimensional skeletal structure is reformed.
Properties of solid electrolyte:
this is,
When making a storage battery, treat it as a melt and treat it as a melt.
Storage battery ・ Can be used as a solid during operation,
It is said to indicate that it is a solid electrolyte.
Evaluate ionic conductivity:
Using pellets obtained by crushing Li (FSA) (SN) 2 single crystals and molding under pressure,
Ion conductivity was evaluated by the AC impedance method.
Activation of ionic conduction:
As a result, the ionic conductivity is
It becomes 10-4Scm-1 near room temperature (30 ℃).
10-5Scm-1 even under low temperature conditions (-20 ℃)
It showed an extremely high value.
further,
The activation energy (Ea) of ionic conduction is 28 kJmol-1.
Lithium-ion transport number (tLi +) is 0.95, etc.
It was found that “characteristics comparable to those of sulfide-based ceramic electrolytes can be obtained.”
Manufacture all-solid-state batteries:
Based on these results, the research group produced a thin-film all-solid-state battery using Li (FSA) (SN) 2 as a solid electrolyte.
The process is as follows.
First, the melt obtained by heating Li (FSA) (SN) 2 is dripped on the positive electrode LiCoO2 thin film.
Next, the metal Li to be the negative electrode is gently placed on the dropped Li (FSA) (SN) 2 melt.
And if it crystallizes by natural cooling,
An all-solid-state battery using Li (FSA) (SN) 2 as a solid electrolyte is completed.
All-solid-state battery charge / discharge test:
The charge / discharge test of the prepared thin-film all-solid-state battery was performed.
As a result, the discharge capacity decreases with each cycle.
It was confirmed that the discharge capacity at the 100th cycle maintained 90% of the initial discharge capacity.
EE Times Japan