Tokyo Tech: Double the battery capacity of all-solid-state batteries: Interface impurity control
-Increased EV cruising range and expanded stationary storage-
2021.01.26
The point:
Discovered that creating a clean interface free of impurities doubles the battery capacity of an all-solid-state battery
The lithium distribution and crystal state near the interface were clarified by synchrotron radiation X-ray diffraction measurement.
Overview:
Professor Ichisugi, Faculty of Materials Science and Engineering, Tokyo Institute of Technology,
Hideyuki Kawashita, Assistant Professor, Tohoku University
Senior Researcher Shirasawa, National Institute of Advanced Industrial Science and Technology,
Professor Masaru Shiraki of Nippon Institute of Technology,
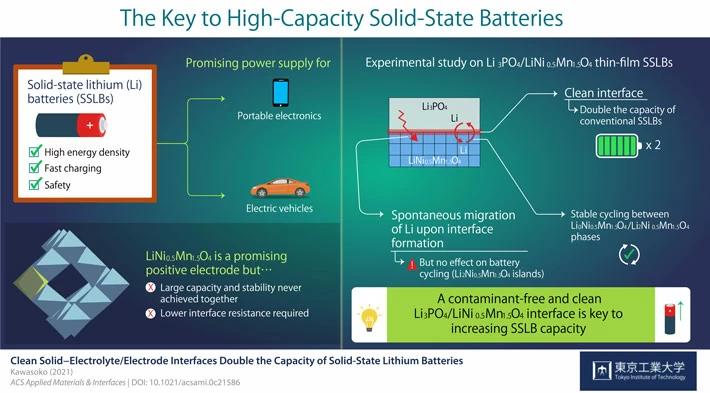
By “controlling impurities at the interface formed by the electrode and the solid electrolyte”, we succeeded in “doubling the capacity of the all-solid-state battery”.
Double the capacity of all-solid-state batteries:
Development goals for all-solid-state batteries include increasing battery capacity and increasing output.
An increase in battery capacity leads to an extension of the usable time of the device.
Higher output makes it possible to charge in a short time and take out a large amount of power instantaneously.
Double capacity:
Creating a clean electrode / electrolyte interface that does not contain impurities
Lithium content doubles.
With Li “2” Ni0.5Mn1.5O4 in the discharged state,
With Li “0” Ni0.5Mn1.5O4 charged
I found that it can be used.
In other words, it means that the capacity has been doubled.
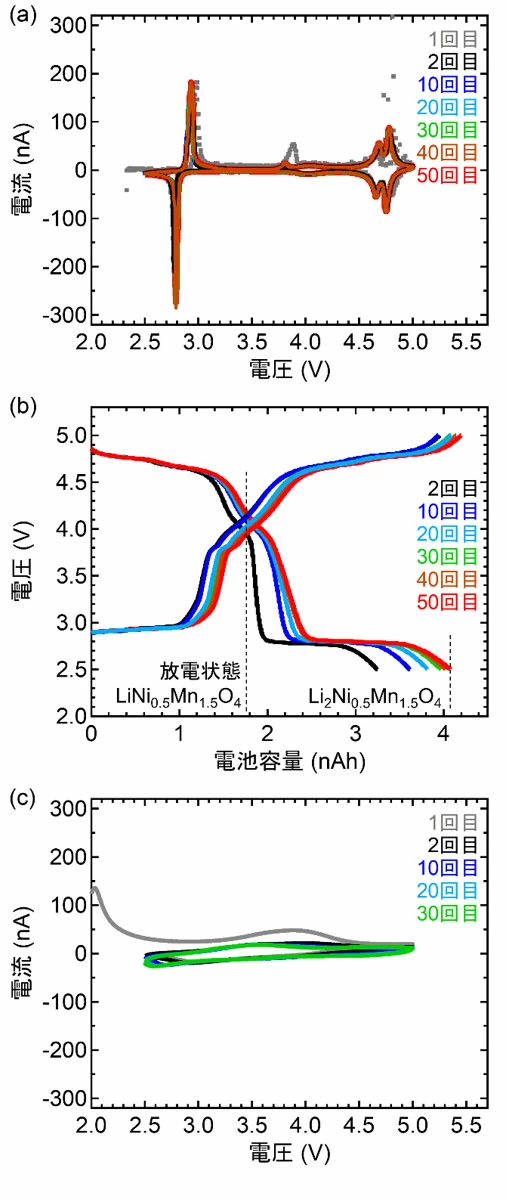
Verification by synchrotron radiation X-ray diffraction measurement:
Furthermore, it was clarified by synchrotron radiation X-ray diffraction measurement that lithium ions move spontaneously during interface formation and Li2Ni0.5Mn1.5O4 is non-uniformly present near the interface.
This research will not only be an important step toward the realization of high-capacity all-solid-state batteries, but will also lead to the establishment of the theory of ion transport at the interface between electrodes and solid electrolytes.
Results of this research:
It was published in the online version of the Journal of the American Chemical Society “ACS Applied Materials and Interfaces” on January 25 (Eastern Time).
the next deployment:
This time, the LiNi0.5Mn1.5O4 all-solid-state battery has doubled the battery capacity.
The new role of the clean electrode / electrolyte interface has been highlighted.
So far, by making a clean interface,
Realization of low interfacial resistance,
In addition to realizing high-speed charging and discharging
This doubling of battery capacity will lead to the expansion of the range of applications for all-solid-state batteries.
Tokyo Institute of Technology News | Tokyo Institute of Technology
https://www.titech.ac.jp/news/2021/048762.html
Keeping a Clean Path: Doubling the Capacity of Solid-State Lithium Batteries
Published: January 26, 2021
Scientists at Tokyo Institute of Technology (Tokyo Tech),
Tohoku University,
National Institute of Advanced Industrial Science and Technology, and
Nippon Institute of Technology,
demonstrated by experiment that
a clean electrolyte/electrode interface is key to realizing high-capacity solid-state lithium batteries.
Their findings
could pave the way for improved battery designs with increased capacity, stability, and safety for both mobile devices and electric vehicles.
solid-state lithium batteries (SSLBs)
SSLBs comprise solid electrodes and a solid electrolyte that exchange lithium (Li) ions during charging and discharging.
Their higher energy density and safety make SSLBs very powerful sources.
However, there are still many technical challenges preventing SSLBs’ commercialization.
For the current study, researchers conducted a series of experiments and gained insight that could take SSLBs’ performance to the next level.
the capacity of SSLBs
Strikingly, the clean interface facilitated the intercalation and deintercalation of Li during charging and discharging of the SSLBs.
As a result, the capacity of SSLBs with a clean interface was twice that of conventional LNMO-based batteries.
Moreover, this study marked the first time stable reversible reactions were found between the L0NMO and L2NMO phases in SSLBs.
Aside from mobile devices,
SSLBs could find a home in electric cars, for which cost and battery durability act as major barriers for widespread commercialization.
The results of this study
provide important insight for future SSLB designs and pave the way for a transition away from fossil fuels and towards more ecofriendly ways of transportation. Keep an eye out for the advent of SSLBs!
Tokyo Tech News | Tokyo Institute of Technology
https://www.titech.ac.jp/english/news/2021/048815.html
Clean Solid–Electrolyte/Electrode Interfaces Double the Capacity of Solid-State Lithium Batteries
Solid-state lithium (Li) batteries
using spinel-oxide electrode materials such as LiNi0.5Mn1.5O4
are promising power supplies for mobile devices and electric vehicles.
Here, we demonstrate stable battery cycling between the Li0Ni0.5Mn1.5O4 and Li2Ni0.5Mn1.5O4 phases with working voltages of approximately 2.9 and 4.7 V versus Li/Li+ in solid-state Li batteries with contamination-free clean Li3PO4/LiNi0.5Mn1.5O4 interfaces.
This clean interface
has the effect of doubling the capacity of conventional battery cycling between the Li0Ni0.5Mn1.5O4 and Li1Ni0.5Mn1.5O4 phases.
We also investigated the structural changes between the Li0Ni0.5Mn1.5O4 and Li2Ni0.5Mn1.5O4 phases during battery cycling.
ACS Applied Materials & Interfaces
https://pubs.acs.org/doi/10.1021/acsami.0c21586
What is synchrotron radiation X-ray diffraction?
http://hep-www.px.tsukuba.ac.jp/TCHoU/LDPPD/event/slide151130/nishibori.pdf