Kyoto Univ icems: Converts CO2 at normal temperature pressure: Porous material (PCP/MOF)
-A new carbon-neutral method-
Kyoto University icems
JEOL RESONANCE
RIKEN
The research group
We have succeeded in developing a new method of converting CO2 into useful porous materials (PCP / MOF) at room temperature and pressure.
Conventional problems:
To convert CO2 into useful materials
Reactions under high temperature and high pressure,
The use of expensive precious metal catalysts was essential.
This new development:
The research group
CO2 is reacted with an organic molecule called amine.
The obtained organic molecule is directly reacted with a metal ion to cause it.
We have developed a new method that can synthesize PCP / MOF at once.
Convert to PCP / MOF:
We devised a combination of amines and metal ions.
It can convert CO2 at normal temperature and pressure into various PCP / MOF.
Structural analysis at the molecular level:
“Synchrotron radiation X-ray diffraction measurement and analysis using solid-state nuclear magnetic resonance spectroscopy (NMR)” was carried out.
1 nm pores are regularly formed inside the molecular level of PCP / MOF.
Its structure turned out to be made up of more than 30% CO2 per weight.
Applications of this solution:
By devising the combination of metal ions and amines,
Synthesis of porous materials with various structures and functions,
CO2 from factory exhaust gas containing a lot of impurities, etc.
It is expected that the target of recycling will be expanded.
What is a porous material?
A solid that has innumerable microscopic cells (pores) inside.
An example is “activated carbon and zeolite in water purifiers and air purifiers.”
In recent years, research on porous materials has been developing more and more.
It is used in a wide range of fields from energy storage to gas separation.
Focus on PCP / MOF:
In this study, we focused on his PCP / MOF, which is a porous material.
PCP / MOF has a “jungle gym-like structure consisting of metal ions and organic compounds (crosslinkable coordination bonds)”.
Since its inception in the late 90’s, more than 90,000 types of PCP / MOF have been developed.
Some of them have been put to practical use for semiconductor gas storage.
PCP / MOF synthesis using CO2 as a raw material:
However, none of them were made from CO2 as a raw material.
The reason for this is that “a method for easily producing crosslinkable coordinates from CO2, which is suitable for PCP / MOF synthesis” has not been searched.
News | Kyoto University icems
https://www.icems.kyoto-u.ac.jp/news/6311/
Succeeded in converting carbon dioxide into a porous material at normal temperature and pressure | RIKEN
https://www.riken.jp/press/2021/20211008_2/index.html
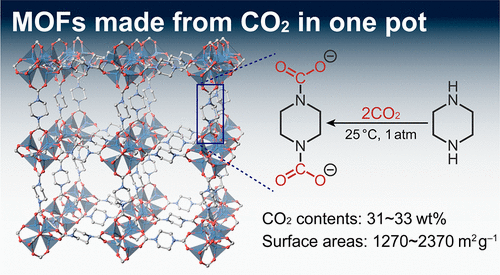
One-Pot, Room-Temperature Conversion of CO2 into Porous Metal–Organic Frameworks
The conversion of CO2 into functional materials under ambient conditions
is a major challenge to realize a carbon-neutral society.
Metal–organic frameworks (MOFs)
have been extensively studied as designable porous materials.Despite the fact that CO2 is an attractive renewable resource,
the synthesis of MOFs from CO2 remains unexplored.
Chemical inertness of CO2
has hampered its conversion into typical MOF linkers such as carboxylates without high energy reactants and/or harsh conditions.Here,
we present a one-pot conversion of CO2 into highly porous crystalline MOFs at ambient temperature and pressure.Cubic [Zn4O(piperazine dicarbamate)3]
is synthesized via in situ formation of bridging dicarbamate linkers from piperazines and CO2 and shows high surface areas (∼2366 m2 g–1) and CO2 contents (>30 wt %).
Whereas the dicarbamate linkers
are thermodynamically unstable by themselves and readily release CO2,
the formation of an extended coordination network in the MOF lattices
stabilizes the linker enough to demonstrate stable permanent porosity.
Journal of the American Chemical Society