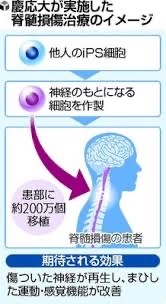
Keio University: Transplanting iPS-derived cells into patients with SCI
-“No safety issues”-
Keio University:
For patients with spinal cord injuries
The cells that are the source of nerves, which were created from iPS cells, were transplanted.
The world’s first transplantation of iPS cells to patients with SCI,
iPS cells for patients with SCI:
As a result of a third-party expert evaluating the progress of the patient who received the transplant,
“The view that there is no safety problem at this time” was summarized.
In response to this, the group will proceed with a second transplant.
NHK | Medical
https://www3.nhk.or.jp/news/html/20220330/k10013558681000.html
Університет Кейо: трансплантація клітин, отриманих з iPS, пацієнтам з SCI
– “Немає проблем з безпекою”-
Університет Кейо:
Для пацієнтів з травмами спинного мозку
Клітини, які є джерелом нервів, створені з iPS-клітин, були пересаджені.
Перша в світі трансплантація клітин iPS пацієнтам з SCI,
Клітини iPS для пацієнтів із SCI:
У результаті стороннього експерта, який оцінює прогрес пацієнта, якому було проведено трансплантацію,
«Погляд на те, що наразі немає проблем із безпекою», було підсумовано.
У відповідь на це група приступить до другої пересадки.
NHK | Медичний
Experts okay Japanese team’s use of iPS cells for spinal cord injuries
NHK has learned that a third-party committee of experts
has found no safety problems so far with a procedure to repair spinal cord injuries by transplanting cells derived from induced pluripotent stem cells, or iPS cells.
A team led by Okano Hideyuki Nakamura Masaya,
both professors at Keio University,performed the world’s first procedure in December.
They transplanted iPS-derived nerve cells into a patient whose spinal cord had been damaged.
The team submitted data on the patient to the Independent Data Monitoring Committee.
The committee concluded on Tuesday
that the patient has so far not shown any grave side effects, and gave the go-ahead for a second clinical trial.The committee did not assess the medical effect of the transplant, as it plans to monitor this aspect over the course of one year.
The team plans to transplant iPS-derived cells into four patients.
They will start accepting applications next month for their second clinical trial.
NHK WORLD-JAPAN News