Japan LIB: Sumitomo Chemical uses aluminum negative electrode material for higher performance: Controls volume expansion / contraction during charge / discharge
Japan LIB:
2020.05.13
Tohoku University / Sumitomo Chemical:
We solved the “problem of using aluminum as the LIB negative electrode material” and clarified the mechanism.
and found that the volume expansion and contraction of the aluminum negative electrode can be controlled during LIB charging and discharging.
We will try to simplify the battery manufacturing process and improve performance in the future.
Conventionally, carbon-based materials have been used for the negative electrode of LIB.
Introducing new materials for higher capacity.
Parts / Materials |
https://www.netdenjd.com/articles/-/232282
Tohoku University and Sumitomo Chemical Reveal a New Mechanism for Preventing Deterioration of Aluminum Anode during Cyclic Battery Reactions
Apr. 27, 2020
Institute for Materials Research, Tohoku University
Sumitomo Chemical, Co., Ltd.
Summary
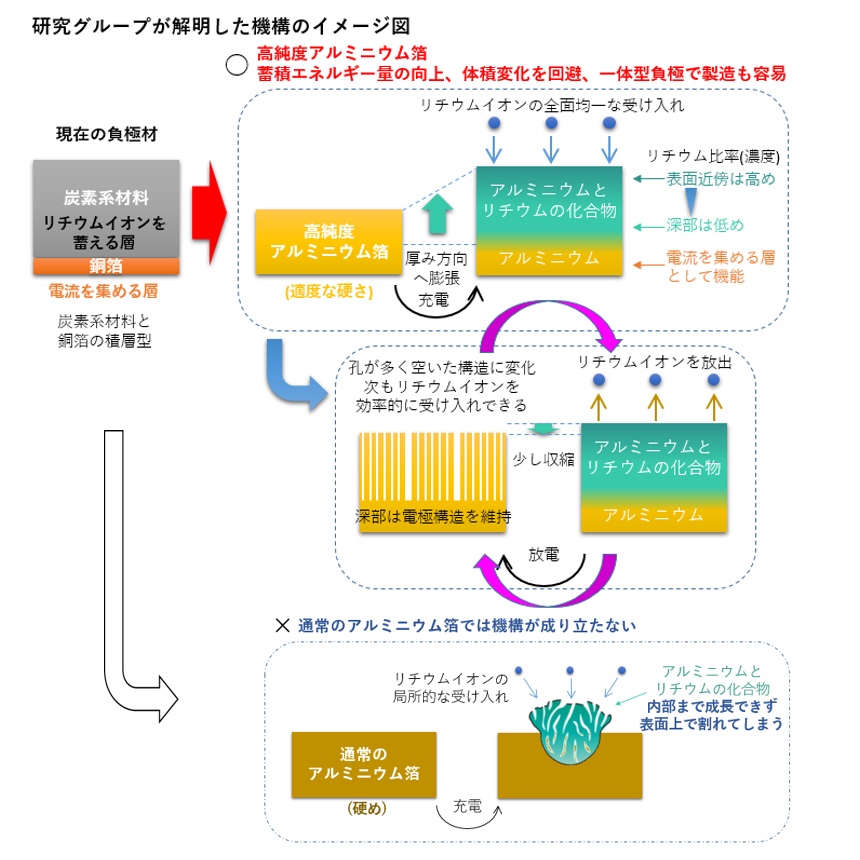
Discovered that the use of high purity aluminum foil can successfully control the huge volume expansion/contraction of high capacity aluminum anodes during charge/discharge processes, and clarified its mechanism.
Determined that high purity aluminum foil
can be an “integrated anode”, which can replace two components of the conventional graphite anode,
i.e., a layered structure of carbon material (that accommodates lithium ions) and copper foil (that functions as a substrate to collect current) as it plays the roles of both components.
Contributed to higher performance and significant simplification of the battery manufacturing process.
Abstract
Research Assistant Professor Hongyi Li and Professor Tetsu Ichitsubo et al. at Institute for Materials Research, Tohoku University and researchers at Sumitomo Chemical
have jointly undertaken the research and development of novel anodes for higher capacity of lithium-ion rechargeable batteries since April 2019.
This joint research group has successfully elucidated a new mechanism for circumventing the huge volume strain during charge/discharge battery reactions with the use of a high purity aluminum foil alone as anode.
Lithium-ion rechargeable batteries
consist of four components: a cathode, an anode, an electrolyte, and a separator film.
Lithium ions move between the cathode and anode when the battery is charged or discharged; when charging,
the anode takes in the lithium ions released by the cathode, and the reverse when discharging. Carbon-based materials are currently the mainstream for anodes.
However, the use of silicon or metals such as tin and aluminum, has been considered as promising anode materials for high energy-density batteries because these materials can store three to ten times more lithium ions than the same weight carbon-based materials.
Despite the higher capacity for absorbing more lithium ions, the application of metal anodes has been deadlocked because their huge volume changes (two to four times) can destroy the anode’s structure.
News Releases | SUMITOMO CHEMICAL
https://www.sumitomo-chem.co.jp/english/news/detail/20200427e.html
Circumventing huge volume strain in alloy anodes of lithium batteries Article
Published: 13 April 2020
Hongyi Li, Takitaro Yamaguchi, […]Tetsu Ichitsubo
Nature Communications volume 11, Article number: 1584 (2020) Cite this article
Abstract
Since the launch of lithium-ion batteries, elements (such as silicon, tin, or aluminum) that can be alloyed with lithium have been expected as anode materials, owing to larger capacity.
However, their successful application has not been accomplished because of drastic structural degradation caused by cyclic large volume change during battery reactions.
To prolong lifetime of alloy anodes, we must circumvent the huge volume strain accompanied by insertion/extraction of lithium.
Here we report that
by using aluminum-foil anodes, the volume expansion during lithiation can be confined to the normal direction to the foil and,
consequently, the electrode cyclability can be markedly enhanced.
Such a unidirectional volume-strain circumvention requires an appropriate hardness of the matrix and a certain tolerance to off-stoichiometry of the resulting intermetallic compound, which drive interdiffusion of matrix component and lithium along the normal-plane direction.
This metallurgical concept
would invoke a paradigm shift to future alloy-anode battery technologies.
Nature Communications