COVID-19: ECMO smaller and lighter, clinical trial started: National Cardiovascular Research Center (NCVC)
National Cardiovascular Research Center:
On April 10, we reduced the size and weight of the ECMO heart-lung machine, ECMO, which is used to save patients with new severe corona pneumonia.
Develop a new model and start “clinical trials (clinical trials) aimed at practical use as medical devices”.
To be portable,
Easy to use in emergency,
The feature is that it is hard to form thrombus and can be used for a long time.
Norio Fukushima Transplantation Medical Director:
“In the future, to aim for use in a doctor helicopter,” he said. Aim for practical use in 3 to 4 years.
Kyodo News
https://headlines.yahoo.co.jp/hl?a=20200410-00000178-kyodonews-soci
Artificial heart-lung machine BR13030
Acute severe heart failure / Acute severe respiratory failure patient / Assisted circulation
Safety and efficacy test (NCVC-ECMO_01)
April 10, 2020
National Cardiovascular Research Center:
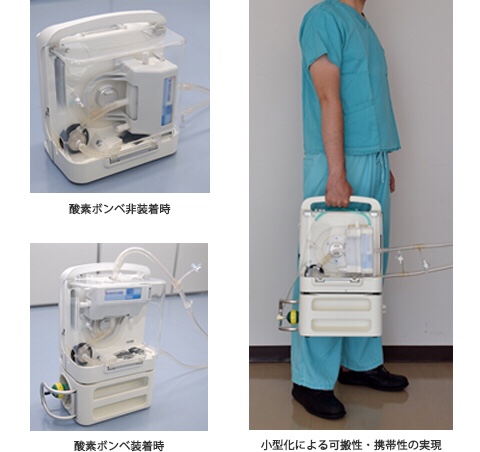
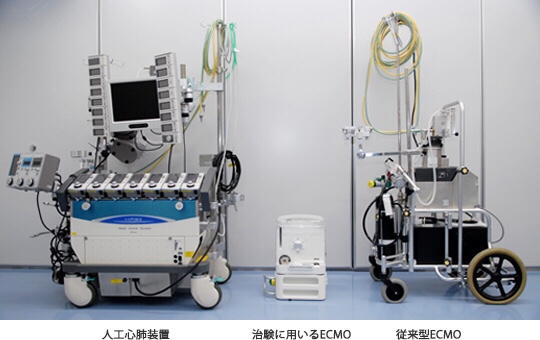
We have developed the BR13030 (Fig. 1), a next-generation cardiopulmonary system that is compact and lightweight and can be used for a long time.
March 10, 2020 / Clinical trial led by physician
National Cardiovascular Research Center
Osaka University Hospital,
Kansai Medical University General Medical Center
It will be implemented at three facilities.
It is also expected to be an effective treatment for severe respiratory failure
due to COVID-19, SARS, MARS, H5N1 avian influenza, etc.
Outline of the device: (Fig. 1)
This device is compact and lightweight (29 x 20 x 26 cm, 6.6 kg) and can be easily carried.
(Easy mounting)
Emergency response is achieved by loading a dedicated circuit unit into a multifunctional integrated / microminiature drive unit.
Ready-to-use system, fast startup in less than 4 minutes.
(safety)
Built-in battery and removable oxygen cylinder, can be used for 1 hour / continuous use.
It also supports emergency out-of-hospital installation, such as during transportation by ambulance.
This device uses an excellent antithrombotic technology (developed by the artificial organ part).
Minimizes anticoagulant therapy and protects against thrombotic and bleeding complications.
(Long-term durability)
Results of continuous cardiopulmonary support for 2 weeks (4 cases) / 4 weeks (3 cases) after wearing in long-term animal experiments.
In all cases, the scheduled period was successfully completed.
In this study, the efficacy of BR13030 was confirmed and is expected to contribute to the survival of critically ill patients.
Press Release | National Cardiovascular Research Center
http://www.ncvc.go.jp/pr/release/20200410_press.html
National Cerebral and Cardiovascular Center