Sapporo Medical Univ: Medical examination of spinal cord injury start-Product for regenerative medicine “Stemilac injection”
Sapporo Medical University Hospital:
Nipro Corporation:
Products for regenerative medicine: “Stemilac injection” (general name: human (self) bone marrow-derived mesenchymal stem cells)
We will start medical treatment of spinal cord injury using “Stemilac injection” jointly developed.
It will start as soon as the supply system for such regenerative medicine products is established.
Planned after the middle of May.
What is the “Stemilac Injection” product for regenerative medicine etc?
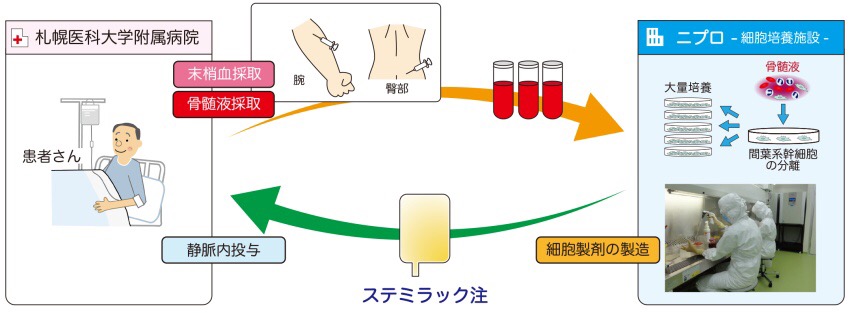
“Stemilac injection” uses mesenchymal stem cells (0.1% in the patient’s bone marrow).
It is a product of regenerative medicine etc. which culture and manufacture these cells.
Incubate your own serum:
In other words, the raw material of Stemilac is bone marrow fluid and blood of the patient.
This bone marrow-derived mesenchymal stem cell is cultured up to 10,000-fold (100 million cells) over 2 to 3 weeks.
Then, through safety testing, quality testing, finally commercialized.
The administration takes about 60 minutes for intravenous infusion in peripheral vein.
Notice | Sapporo Medical University Hospital