Figure 1. Picture of the proton conducting membrane created in this research on a molecular scale
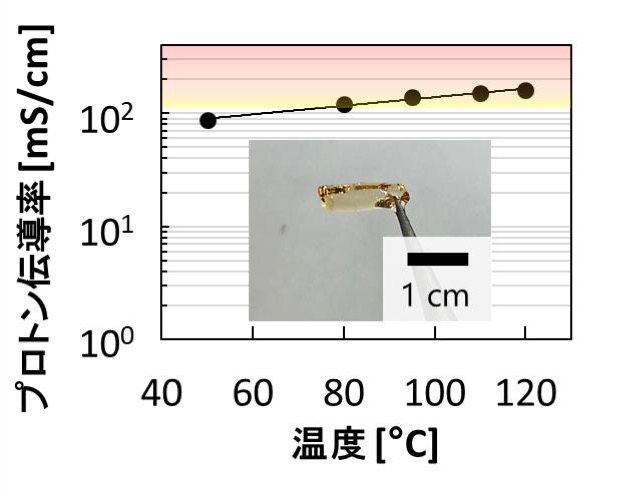
Fig. 2. Photograph of the membrane obtained in this study and proton conductivity without temperature relative to temperature
Nagoya Univ: Creation of Fuel Cell Membrane for Next-Generation FCV: Journal of Materials Chemistry A
Demonstrate high performance over conventional membranes even without humidification
May 29, 2019
The Nagoya University Research Group, in collaboration with Toyota, has created a new fuel cell membrane for use in next-generation FCVs.
New fuel cell:
Gaseous hydrogen is a positively charged hydrogen ion (proton).
This proton reacts with oxygen to generate electricity.
New fuel cell: power generation performance:
The power generation performance of a fuel cell largely depends on the “proton conductivity of the proton conducting membrane (the separation of hydrogen and oxygen, the role of proton transport)”.
Conventional fuel cell: Requires humidification system
The proton conducting membrane (conventional membrane) currently used in FCV requires a humidification system to achieve high proton conductivity. Without the humidification system, the fuel cell can not operate (generate power).
This fuel cell: no need for humidification system
In this joint research, polymer (Note 4) and non-volatile (5) acidic liquid are combined. And we created a new membrane material.
New Fuel Cell Membrane: Proton Transportability
It has been confirmed that the performance of the conventional membrane is equal to or higher than that of the proton transport capacity shown under humidification (proton conductivity membrane (140 mS / cm at 95 ° C.). The proton conductivity was almost zero above (100 ° C).
However, new fuel cell membranes are different from conventional membranes. It has been confirmed that high proton conductivity (160 mS / cm at 120 ° C, 220 mS / cm at 160 ° C) of 100 mS / cm or more is shown even at 100 ° C. or more under no humidity.
The research results were published on May 3, 2019 in the online version of the journal “Journal of Materials Chemistry A” of the Royal Chemical Society of the United Kingdom as a bulletin (Communication).
http://www.nagoya-u.ac.jp/about-nu/public-relations/researchinfo/upload_images/20190529_engg001.pdf