Tohoku University: Therapeutic effect on cerebral infarction: Muse cell preparation “CL2020”
-Using universal cells discovered by Tohoku University-
Tohoku University Hospital:
Since 2018, clinical trials using Muse cells are underway at Tohoku University Hospital.
After the onset of cerebral infarction, clinical trials were conducted on “35 patients who were bedridden within 4 weeks and needed assistance”.
35 subjects with cerebral infarction:
“Everyone needs assistance in walking and daily life.”
“Because they were bedridden or incontinent, They need long-term care.”
The subjects were divided into two groups.Twenty-five people received CL2020
Ten people received placebo.A double-blind comparative study was conducted in which the two groups were administered.
Recovery status after 12 weeks:
The group receiving CL2020 showed a remarkable recovery.
Twelve weeks after administration, the rate of recovery to the level of independence in daily life is as high as 40%.
On the other hand, the recovery rate of the placebo-administered group remains at 10%.
Recovery status after 52 weeks (1 year):
In 52 weeks (1 year), it reached 68.2%.
The rate of recovery to the state of returning to work also reached 31.8%.On the other hand, the rate of returning to work in the placebo group was zero.
15 out of 22:
About 15 out of 22 people who received the infusion preparation containing “Muse cells”
One year after the administration, They said, “Recovered to a state where transportation can be used without assistance.”
Seven of these 15 have returned to work.
Clinical trials with Muse cells:
This cerebral infarction is the first time that clinical trials using “Muse cells” have revealed results.
What are Muse cells?
Amyotrophic lateral sclerosis = ALS and
New Coro Severe respiratory failure, etc.
No radical cure has been established,Clinical trials are underway for six diseases, and the people concerned are looking forward to it.
KHB East Japan Broadcasting
http://www.khb-tv.co.jp/news/localNews/202105181827022.html
Recovery with just one dose: “Muse cells” announced by Tohoku University
-Potential for cerebral infarction patients, formulation of “a type of stem cell” that everyone has-
Origin of muse cells:
It was discovered in 2007 by Mari Dezawa, a professor at Tohoku University Graduate School.
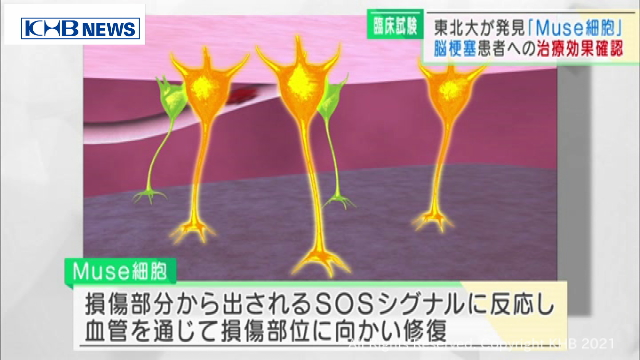
It is a type of stem cell that differentiates into various cells.
Natural cells that exist in everyone’s body.When some kind of abnormality occurs in cells such as organs, it has the property of catching signals and naturally gathering in the affected area to repair them.
Mitsubishi Chemical Holdings:
Life Science Institute (LSII):Treatment with muse cell preparation “CL2020” is underway.
The Mitsubishi Chemical Life Science Institute (LSII) has worked on the formulation.
Muse cell clinical trials are underway in multiple diseases.
New drug approval application:
LSII plans to apply for approval of CL2020 as a new drug within this year.
Manufacturing and sales are expected to be approved as early as next year.
Bungei Online