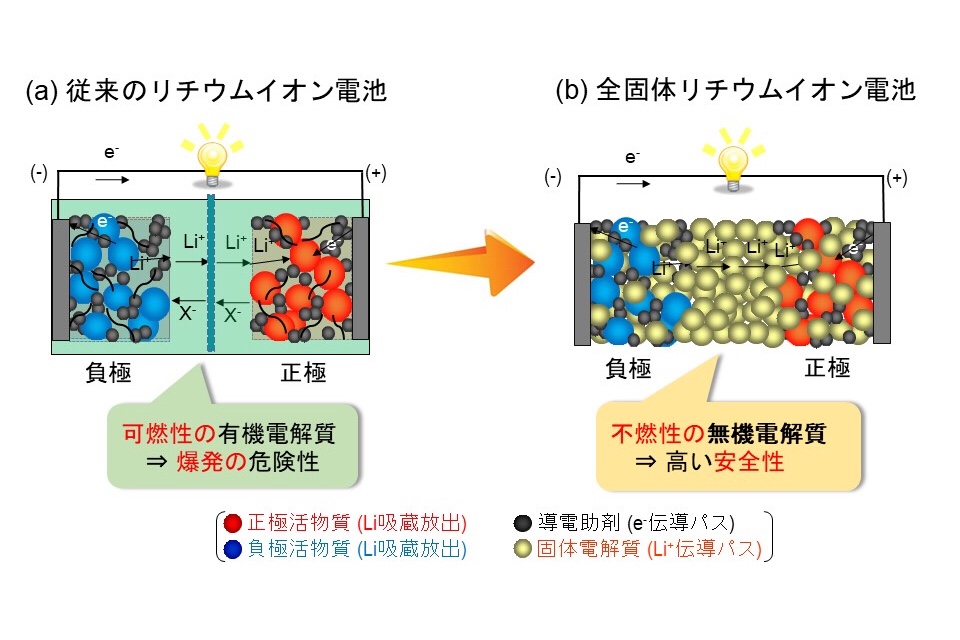
Figure 1. Schematic diagram of a conventional lithium-ion battery (a) and an all-solid-state lithium-ion battery (b).

Figure 2. Exhaustive calculation results for the search for solid electrolyte materials with both high ionic conductivity and high formability.
Nagoya Institute of Technology: Development of new all-solid-state LiB material: Internal density 94%
-Effective in suppressing metallic Li short circuit: Realization of new chloride solid electrolyte and safe in-vehicle battery-
Nagoya Institute of Technology:
We have developed a high moldability chloride solid electrolyte material.
A lithium metal electrode (*2) with high energy density has realized a stable charge/discharge cycle.
Chloride solid electrolyte material:
Set the physical property indexes required for solid electrolyte materials.
For the materials in the structural database, we comprehensively calculated and searched for efficient materials.
inside that,
We succeeded in “synthesis under normal temperature and pressure of inert gas” for toxic gases and chloride materials that require high temperature treatment.
In addition, we were able to “reduce the short-circuit phenomenon, which is a problem of lithium metal negative electrodes,” in a process that uses only compacted powder and has a low environmental load.
Solid electrolytes are the key:
The key to achieving high energy density in all-solid-state batteries is the “solid electrolyte in which lithium ions conduct in solids”.
This new material realizes high energy density of all solid state batteries.
Nagoya Institute of Technology
https://www.nitech.ac.jp/news/press/2020/8406.html
Metastable Chloride Solid Electrolyte with High Formability for Rechargeable All-Solid-State Lithium Metal Batteries
Naoto Tanibata*, Shuta Takimoto, Koki Nakano, Hayami Takeda, Masanobu Nakayama, and Hirofumi SumiACS Materials Letters
Abstract
Dense solid electrolytes in all-solid-state Li batteries
are expected to suppress Li dendrite phenomena that prevent the application of high-energy-density Li metal electrodes.
However, voids and cracks in sintered electrolytes still permit short-circuiting due to Li dendrites.
This study aimed to investigate solid electrolytes with high formability in which green compacts can prevent Li dendrites.
Li+ ion migration energies, bulk moduli, and energies above the hull
were comprehensively investigated using first-principles and classical force field calculations as the indicators for ionic conductivity, formability, and thermodynamic stability.
The 231 compounds containing Li and Cl listed in the Materials Project database were studied due to their high polarizability and weak Coulombic interaction with Li+ ions.
Among them, monoclinic LiAlCl4 (LAC, S.G.: P121/c1) was focused on, owing to its low values of all three indicators.
A mechanochemical synthesis
was attempted to prepare the metastable phase, where Li ions occupy the interstitial sites, not just the original sites, because the computation for the migration energy suggested conductive pathways between the original Li sites.
XRD and 7Li-MAS NMR measurements indicated that
the mechanochemically synthesized LAC possessed a monoclinic host structure, while 2.5% Li occupied interstitial tetrahedral sites.
Impedance measurements
showed that the LAC green compacts exhibited an ionic conductivity of 2.1 × 10–5 S cm–1, 20 times higher than the conventional solid-state synthesized LAC at room temperature.
The conductivity
was more than one order of magnitude higher than that of garnet-type Li6.6La3Zr1.6Ta0.4O12 (LLZT), which has been attractive for the application of the sintered body for Li metal electrodes.