CYBERDYNE: HAL, EU medical equipment certification: TÜV Rheinland LGA Products
CYBERDYNE: HAL
On October 8, 2019, the robot suit single joint type for HAL medical treatment was certified as “Compliant with the European Medical Device Directive (MDD)”.
Third-party certification body: TÜV Rheinland LGA Products
With this MDD certification, the medical HAL series has been expanded.
In addition, the indications and treatment scenes will expand, and more patients will benefit from the medical HAL series.
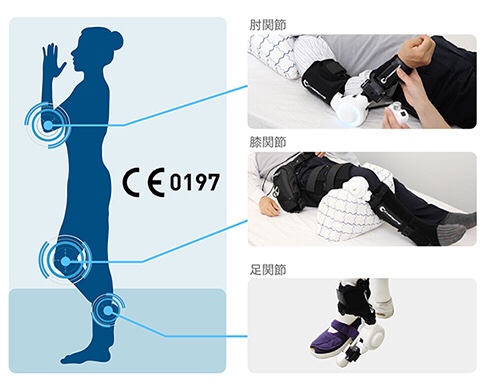
HAL medical single joint type:
Used for cybernics treatment of elbow, knee and ankle joints.
Lightweight and compact, it can be used in various postures according to the wearer’s physical condition.
Cybernics treatment can be started on the bed from an extremely early stage.
MONOist