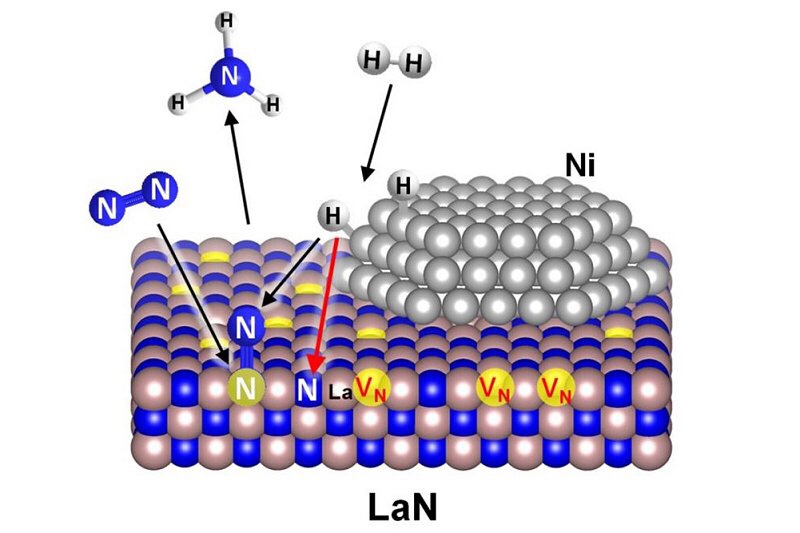
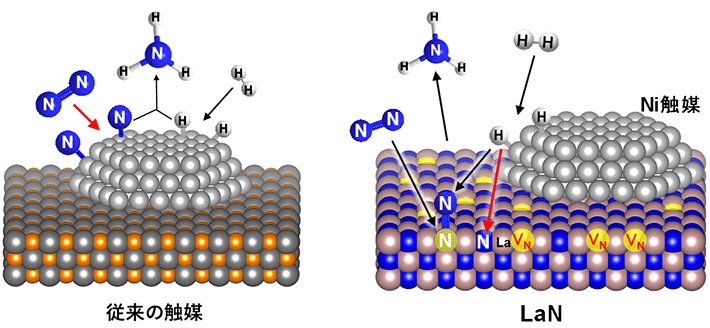
Reaction mechanism of ammonia synthesis on conventional catalyst (left) and developed catalyst Ni/LaN (right). VN is a nitrogen vacancie formed on LaN, and the red arrow is the rate-determining reaction process. Dissociation of N2 occurs not at the metal surface but at the nitrogen vacancies on the LaN surface.
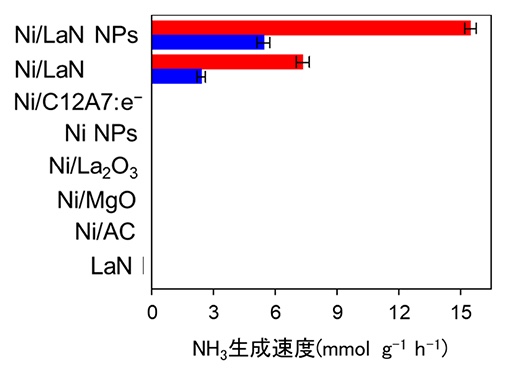
Comparison of ammonia synthesis activity of LaN with Ni fixed and other catalysts (reaction temperature: 400 ℃, pressure: 1 atm (blue), 9 atm (red))
Tokyo Institute of Technology: Successful highly efficient ammonia synthesis: Using Ni/LaN catalyst
Release Date: 20 July, 2020
The points:
- Succeeded in highly efficient ammonia synthesis using nickel (Ni) and lanthanum nitride (LaN) that do not show activity alone
- Achieves high ammonia synthesis activity without the use of precious metals such as ruthenium
- Demonstration of a new concept of using nitrogen vacancies on the surface of LaN as a reaction field
Overview:
Elementary Strategy Research Center, Tokyo Institute of Technology
Professor Emeritus Hosono,
Assistant Professor, Tian-Nan Ye
Kitano Associate Professor
This time ammonia synthesis catalyst:
By combining nickel (Ni) and lanthanum nitride (LaN), which do not show activity by themselves, we have realized an excellent ammonia synthesis catalyst comparable to noble metal catalysts such as ruthenium.
Conventional ammonia synthesis catalyst:
The conventional ammonia synthesis catalysts are
Iron is used in the synthesis under high temperature and high pressure.
Use ruthenium under mild conditions.
Since both metals strongly bind to nitrogen, the reaction took place on the metal.
On the other hand, nickel, which has a very weak bond with nitrogen, has not been used so far because it cannot activate the nitrogen molecule.
In this study:
Activation of hydrogen molecules on nickel,
Activation of nitrogen molecules is performed by nitrogen vacancies on LaN,
By doing each, extremely high ammonia synthesis activity is achieved.
Use of the reaction field called nitrogen vacancies:
It was shown that excellent ammonia synthesis can be realized by using a metal that does not show activity alone.
This is a research result that overturns conventional wisdom.
Results of this research:
All of the catalysts that have been developed in recent years and have a high activity of synthesizing ammonia under mild conditions needed to carry ruthenium, which is a noble metal.
In this research, it is important as a new catalyst technology that does not use rare and expensive ruthenium.
This leads to new possibilities in the ammonia synthesis process.
The research results were published online in the British science journal “Nature” on July 16 (Japan time).
Tokyo Tech News | Tokyo Institute of Technology
https://www.titech.ac.jp/news/2020/047268.html
Vacancy-enabled N 2 activation for ammonia synthesis on an Ni-loaded catalyst
Article
Published: 15 July 2020
Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst
Tian-Nan Ye, Sang-Won Park, […]Hideo Hosono
Nature volume 583, pages391–395(2020)Cite this article
Abstract
Ammonia (NH3) is pivotal to the fertilizer industry and one of the most commonly produced chemicals1.
The direct use of atmospheric nitrogen (N2)
had been challenging, owing to its large bond energy (945 kilojoules per mole)2,3, until the development of the Haber–Bosch process.
Subsequently, many strategies
have been explored to reduce the activation barrier of the N≡N bond and make the process more efficient.
These include using alkali and alkaline earth metal oxides as promoters to boost the performance of traditional iron- and ruthenium-based catalysts4,5,6 via electron transfer from the promoters to the antibonding bonds of N2 through transition metals7,8.
An electride support further lowers the activation barrier because its low work function and high electron density enhance electron transfer to transition metals9,10.