Nagoya Institute of Technology: CO2 decomposition by sunlight: Photocatalytic single-walled carbon nanotubes
-Successful development of a photocatalyst that is easy to synthesize-
Nagoya Institute of Technology:
This time, the Nagoya Institute of Technology research group has developed a solar CO2 reduction catalyst.
This solar CO2 reduction catalyst efficiently absorbs visible light.
Solar CO2 reduction catalyst:
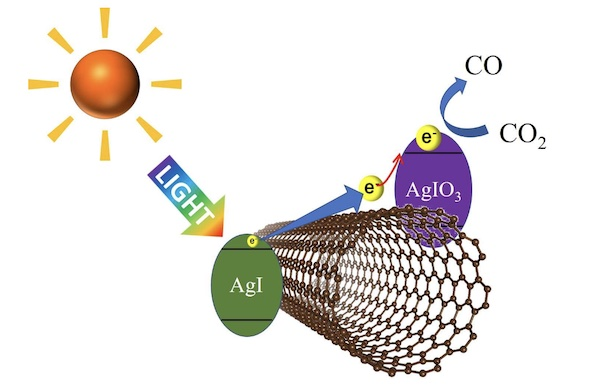
Iodide (AgI) crystallites that absorb visible light,
Microcrystals of iodic acid (AgIO3) that reduces CO2,
It was uniformly dispersed and supported on single-walled carbon nanotubes.
Easy-to-synthesize photocatalyst:
This synthesis method
“Single-walled carbon nanotubes containing iodine molecules”,
“Just immerse in an aqueous silver nitrate solution”
Efficiently absorbs visible light.
It’s a very simple method.
–Smart Japan
https://www.itmedia.co.jp/smartjapan/articles/2105/18/news051.html
Developed photocatalyst: Iodine in nanotubes kills CO2
~ Decompose global warming gas with carbon nanotubes ~
Decomposes global warming gas
Nagoya Institute of Technology
Professor Shinji Kawasaki,
Assistant Professor Yosuke Ishii,
Research group:
We have developed a “photocatalyst that uses visible light with high light intensity”.
Using single-walled carbon nanotubes,
Developed a photocatalyst using visible light,
This decomposes the global warming gas.
Photocatalytic single-walled carbon nanotubes:
These carbon nanotubes decompose carbon dioxide, which is a global warming gas.
This research directly contributes to solving environmental problems.
Solar CO2 reduction catalyst:
Efficiently absorbs visible light-AgI
Efficiently reduces visible light-AgIO3
Both microcrystals were “uniformly dispersed and supported on single-walled carbon nanotubes” (Fig. 1).
Method for synthesizing photocatalytic complex:
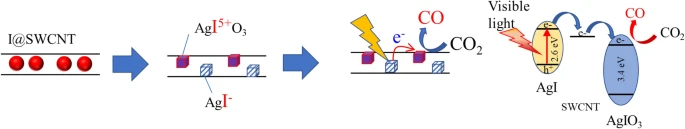
This photocatalytic complex synthesis method is unique and very easy.
Simply immerse iodine molecules and single-walled carbon nanotubes in an aqueous silver nitrate solution.
By the disproportionation reaction, microcrystals of two kinds of iodine compounds can be uniformly supported on the nanotubes at the same time (Fig. 2).
The synthesis cost can be suppressed, and widespread practical application can be expected.
Fabrication of flexible transparent photocatalytic electrode:
In addition, we have already composited nanotubes.
In other words
Simply apply the photocatalytic complex onto an insulating transparent polymer
Flexible transparent photocatalytic electrodes (Fig. 3) can be manufactured.
Devices for CO2 reduction:
It can be installed in various places.
It is expected to be applied as a device to reduce CO2, a global warming gas.
This research result:
It was published in Nature Research’s Scientific Reports magazine on May 12, 2021 (Japan time).
Nagoya Institute of Technology
https://www.nitech.ac.jp/news/press/2021/8970.html
One-step synthesis of visible light CO 2 reduction photocatalyst from carbon nanotubes encapsulating iodine molecules
Abstract
We describe the synthesis and visible-light CO2 photoreduction catalytic properties of a three-component composite consisting of AgI, AgIO3, and single-walled carbon nanotubes (SWCNTs).
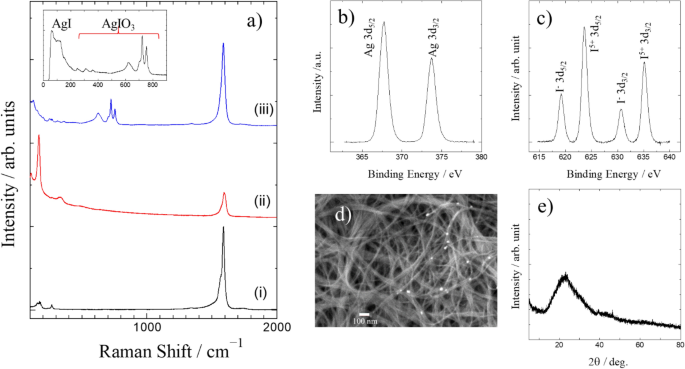
The catalyst is synthesized by immersing SWCNTs encapsulating iodine molecules in AgNO3 aqueous solution, during which neutral iodine (I2) molecules encapsulated in SWCNTs transform disproportionately to I5+ (AgIO3) and I− (AgI), as revealed from the characterization of the composite by Raman spectroscopy, X-ray diffraction, and X-ray photoelectron spectroscopy.
In addition,
photoirradiation experiments using a solar-simulator (AM1.5G) showed thatthe obtained three-component composite works as a CO2 photoreduction catalyst under visible light despite the wide band gap of AgIO3, suggesting possible transfer of the visible light-excited electron from AgI via SWCNTs.
Scientific Reports