JAEA: Bone adsorbs strontium: Developed highly carbonated apatite
Japan Atomic Energy Agency:
University of Tokyo:
The research group announced on February 4 that it has developed an inexpensive and highly efficient metal adsorbent made from waste pork bones.
“Carbonic acid contributes to the mechanism by which bone adsorbs metals such as strontium.”
Utilization of food waste:
The pork bone gala of food waste was soaked in a household aqueous solution of baking soda (sodium hydrogen carbonate).
“Highly carbonated apatite” was created, analyzed and developed.
Nuclear industry newspaper
https://www.jaif.or.jp/journal/japan/6461.html
Waste pork bone becomes a harmful metal adsorbent
-Realize new metal adsorption technology-
We have developed an inexpensive and high-performance metal adsorption technology using waste materials.
Utilizing pork bone gala:
The experiment was conducted using pork bones, which is a food waste, and baking soda (sodium hydrogen carbonate), which is also used as a food additive.
After pressurizing and heating the pork bones
When immersed in an aqueous solution containing baking soda,
Confirmed the formation of a white structure that maintained the shape of the bone (Fig. 1)
Structure composition and microstructure analysis:
The composition and microstructure of this structure were investigated.
It turned out that it was composed of nanometer-sized carbonated apatite crystals.
This carbonated apatite contains more carbonic acid than normal bone.
Carbonated apatite crystals:
Increasing the concentration of the aqueous solution of baking soda to be immersed increased the amount of carbonic acid.
In other words, it was found that “when immersed in baking soda, high carbonic acid-containing apatite containing a large amount of carbonic acid is formed.”
Analysis of strontium adsorption performance:
The prepared carbonate apatite was stirred in an aqueous solution of strontium (concentration of 0.1 mol / L).
After that, the strontium remaining in the solution was measured to investigate the strontium adsorption performance.
Evaluation of strontium adsorption:
Carbonated apatite adsorbed 99% strontium in solution within 3 minutes.
The adsorption performance was investigated in more detail.
The partition coefficient Kd value [5] of carbonate apatite is 274,780 mL / g.
It was 250 times higher than that of untreated bone (Fig. 2, left).
Adsorption performance of cadmium and lead:
We also investigated the adsorption performance of carbonate apatite on cadmium and lead.
As a result, it was found that “carbonated apatite adsorbs cadmium and lead with high efficiency”.
[Future prospects]
Using pork bones from food waste,
In a simple process,
Has high performance heavy metal adsorption performance,
We have succeeded in developing an adsorbent.
In addition to purifying contaminated water, this material can be embedded in soil to prevent pollutants from spreading into the environment, such as groundwater.
Countermeasures against environmental pollution problems:
It is expected to be used for the purpose of maximizing the possibility of human intake of harmful metals.
Furthermore, “use as an adsorbent for the purpose of recovering useful metals” is expected.
Patents are pending in Japan and overseas in connection with this achievement.
Japan Atomic Energy Agency: Press Release
https://www.jaea.go.jp/02/press2020/p21020401/
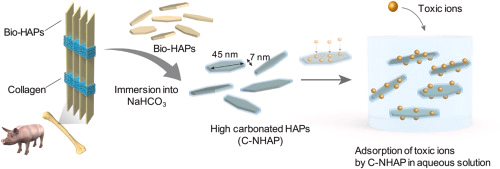
Carbonated nanohydroxyapatite from bone waste and its potential as a super adsorbent for removal of toxic ions
Abstract
The effective, low-cost decontamination of toxic metals
is critical for addressing global health risks, reducing environmental pollution, and building a sustainable future.
Here, we developed
an eco-friendly hydroxyapatite nanocrystal adsorbent made from bone waste that can effectively capture 90Sr.
Highly carbonated nanohydroxyapatite (C-NHAP) crystals with a negatively charged surface
were obtained by simply immersing pig bone in an aqueous solution of sodium hydrogen carbonate (NaHCO3).
Fourier transform infrared spectra showed that
the C-NHAP was highly carbonated and that the amount of introduced carbonate ions (CO32−) increased with increasing NaHCO3 concentration in the immersion solution.
With increasing amount of CO32− ions introduced into the C-NHAP, it exhibited a greater ion-adsorption performance. The distribution coefficient (Kd = 24,780 mL g−1) of the C-NHAP for Sr2+ was approximately 20 and 250 times greater than those of clinoptilolite and untreated bone, respectively.
The C-NHAP also exhibited high adsorption capacity (Qe = 125 mg g−1) for Sr2+.
The extended X-ray absorption fine structure spectra
showed that the CO32− sites in C-NHAP played an important role in its high adsorption performance.
The C-NHAP exhibited high adsorptivity for Cd2+, Pb2+, and Cu2+.
The C-NHAP prepared from bone waste
is an eco-friendly, high-performance, low-cost material that should be useful in environmental pollutant removal and food waste disposal.
ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S2213343721000920