💊FDA PHARMA today
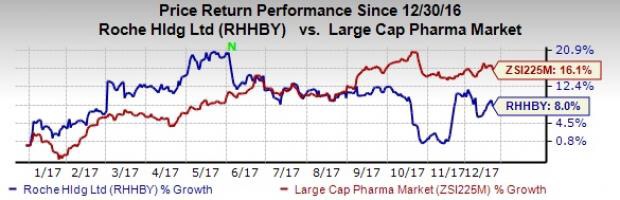
Roche – FDA approves Roche’s Perjeta (pertuzumab) for adjuvant treatment of specific type of early breast cancer.
Roche today announced the US Food and Drug Administration (FDA) has approved Perjeta® (pertuzumab), in combination with Herceptin® (trastuzumab) and chemotherapy (the Perjeta-based regimen), for adjuvant (after surgery) treatment of HER2-positive early breast cancer (eBC) at high risk of recurrence.
https://www.roche.com/media/store/releases/med-cor-2017-12-21b.htm
Roche – European Commission approves Roche’s Alecensa (alectinib) as first-line treatment in ALK-positive lung cancer
https://www.roche.com/investors/updates/inv-update-2017-12-21.htm
Roche’s Perjeta Gets FDA Nod for Post Surgery Breast Cancer – December 21, 2017 – Zacks.com
https://www.zacks.com/stock/news/286709/roches-perjeta-gets-fda-nod-for-post-surgery-breast-cancer
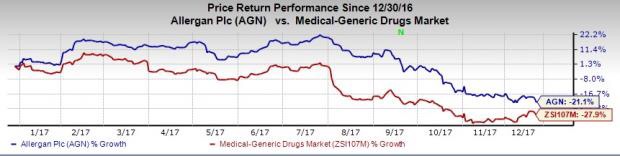
Allergan/Paratek’s Acne Candidate NDA Filing Accepted by FDA
Allergan plc AGN along with partner Paratek Pharmaceuticals PRTK announced that its new drug application (NDA) for its investigational, once-daily, Seysara (sarecycline) has been accepted for review by the FDA for treatment of patients aged nine years and above with moderate to severe acne vulgaris.
The company expects a response from the FDA in the second half of 2018.