
COVID-19: Therapeutic effect of nafamostat mesylate: confirmed by the University of Tokyo
COVID-19:
2020/7/8 05:00
University of Tokyo Hospital: Associate Professor Doi
A clinical study was conducted on “critically ill patients with COVID-19 who developed pneumonia and needed treatment in the intensive care unit (ICU)”.
COVID-19 therapeutic drug candidate “nafamostat mesilate” and “favipiravir (trade name: Avigan)” were used in combination for treatment.
Conducting clinical trials:
Symptom recovery was seen in 10 of the 11 patients.
It was administered to 11 patients (10 males and 1 female) aged 36 to 75 years who were hospitalized from April 6 to 21.
Observe the clinical course.
Nafamostat mesylate:
Administration of 0.2 mg nafamostat mesylate per kilogram of body weight every 1 hour for about 2 weeks.
Favipira Building:
Favipiravir was administered at 3600 mg per day on the first day and 1600 mg from the second day for about 2 weeks.
Status of medical condition recovery:
Recovery of symptoms was seen in 10 people.
Of the recovered patients,
There are 7 patients who used the ventilator.
Three out of seven required a cardiopulmonary support system (ECMO).
However, the ventilator became unnecessary in about 16 days.
Specific clinical research scale:
Specific clinical studies observing the combined effects are:
Starting in May, the University of Tokyo Hospital,
It starts at 6 facilities in Japan and is held at 8 facilities as of the end of May.
The results were published in the electronic version of the critical care medical journal.
The two drug candidates have different sites of action during the viral growth process.
Therefore, the effect of the combined use is expected.
Nikkan Kogyo Shimbun
https://www.nikkan.co.jp/articles/view/00563690
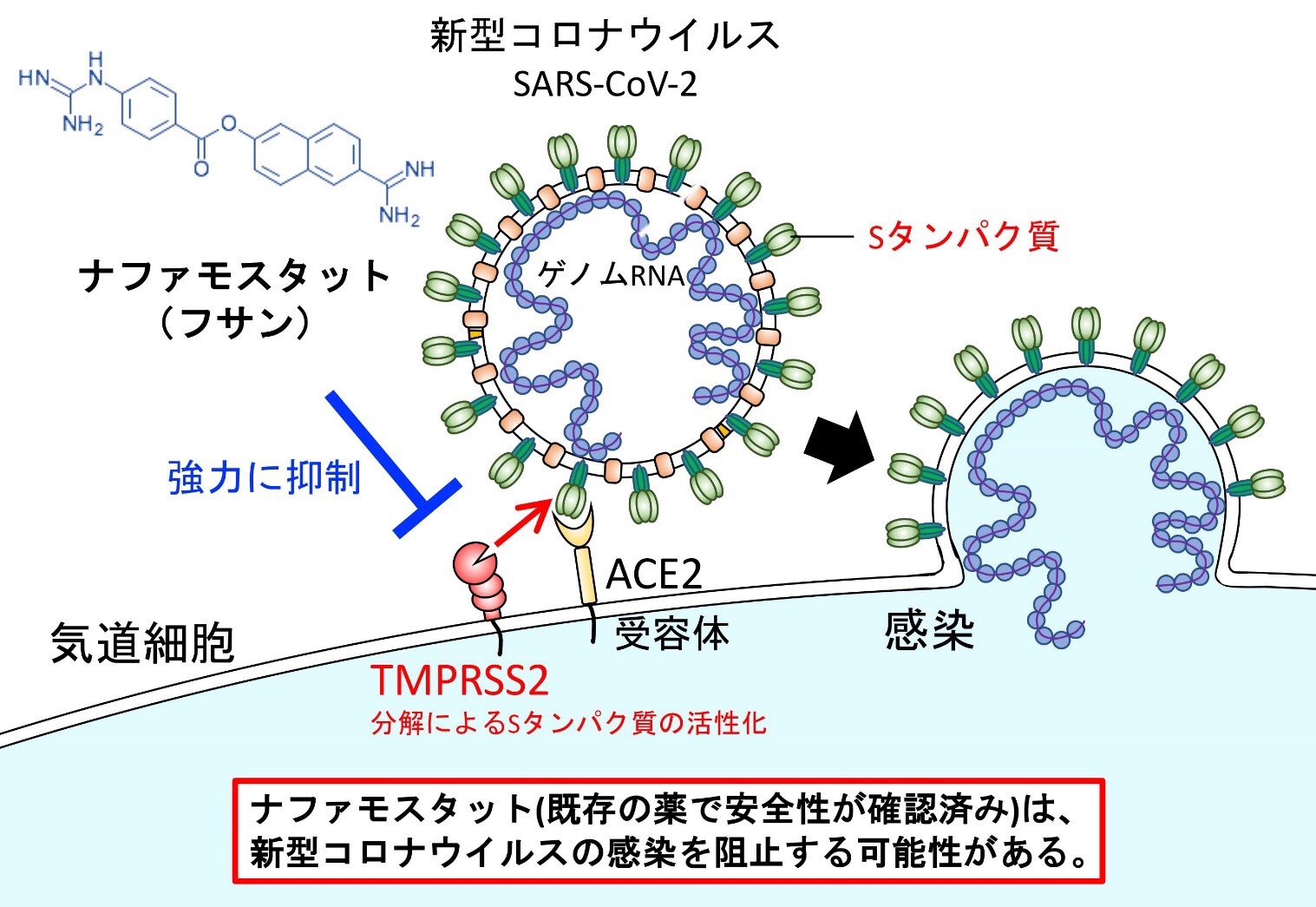
Identification of an existing Japanese pancreatitis drug, Nafamostat, which is expected to prevent the transmission of new coronavirus infection (COVID-19)
March 23, 2020
Nafamostat mesylate (brand name: Fusan), which is the drug used to treat acute pancreatitis,
may effectively block the requisite viral entry process the new coronavirus (SARS-CoV-2) uses to spread and cause disease (COVID-19).
The University of Tokyo announced these new findings on March 18, 2020.
According to the new research,
Nafamostat
can prevent the fusion of the envelope of the virus with host cell surface membranes, the first step in infection with the causative virus SARS-CoV-2.
Nafamostat
can inhibit the membrane fusion at a concentration less than one-tenth that of Camostat mesylate (brand name: Foypan), which was recently identified by a German group as an inhibitor of SARS-CoV-2 infection (Reference 1).
Both Nafamostat and Camostat
were developed in Japan as treatments for pancreatitis and some other diseases.
These drugs
have been prescribed in Japan for many years and have adequate clinical data with regard to safety.
THE INSTITUTE OF MEDICAL SCIENCE, THE UNIVERSITY OF TOKYO
https://www.ims.u-tokyo.ac.jp/imsut/en/about/press/page_00002.html