COVID-19: Japanese Avegan clinical trial, continued in July: Infected population shortage
COVID-19:
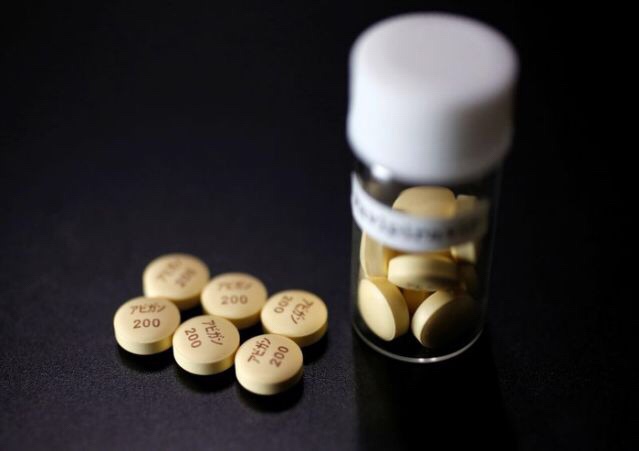
COVID-19 Therapeutic Candidate, Avigan Clinical Trials:
At the end of June, We couldn’t make it in time,
It is expected to continue after July
It turned out on June 8.
This is because the number of clinical trial subjects is decreasing as the number of infected persons decreases.
FUJIFILM:
According to FUJIFILM Holdings, the end date is undecided.
Originally, FUJIFILM/Toyama Kagaku has a schedule to carry out from the end of March to the end of June with the goal of 96 participants.
The plan seems to be delayed due to the sharp decrease in COVID-19 infections.
In the future, we would like to increase the number of hospitals that accept clinical trials and accelerate the work for early approval.
https://this.kiji.is/642679958022423649
Fujifilm says COVID-19 drug research may drag on into July
June 7, 2020, 2:24 pm
TOKYO (Reuters) –
Fujifilm Holdings Corp’s research on Avigan as a potential treatment for COVID-19 may drag on until July, the company said on Sunday,
a further setback in the Japanese firm’s race to find a vaccine.
There is a possibility that clinical trials will continue in July, a Fujifilm spokesman said,
responding to a Nikkei report that any approval will be delayed until July or later, due to a lack of patients for trials.
But researchers have only been able to get around 70% of the patients needed for the trials, and because it takes 28 days to get results,
the process will continue until at least July, the Nikkei business daily said, citing an unnamed source.
https://news.yahoo.com/amphtml/fujifilm-says-covid-19-drug-052439601.html