COVID-19: FUJIFILM to Kuwait Avegan Trial: The sources said
COVID-19:
FUJIFILM Holdings:
On July 18, India’s partner Dr. Reddy’s Laboratories, Fujifilm,
“We will start a clinical trial of the COVID-19 therapeutic agent, Avigan in Kuwait by the end of July,” he said.
We will conduct clinical trials for up to 1,000 people in Kuwait and confirm the effects.
Clinical trials in Kuwait:
FUJIFILM has granted “Dr. Reddy’s Laboratories, a major Indian pharmaceutical company, exclusive development rights to overseas companies,” and will carry out mainly by Dr. Reddy’s.
Johns Hopkins University:
According to the statistics, the total number of infected people in Kuwait exceeds 58,000.
If valid data is obtained in Kuwait, it may be used in Japan.
KYODO communication
https://this.kiji.is/657040464651830369
Fujifilm to start clinical study of COVID-19 drug Avigan in Kuwait
Fujifilm Holdings Corp.
will start a clinical study of the antiviral drug Avigan in Kuwait as early as this month in collaboration with an Indian partner company, sources close to the matter said Saturday.
India’s Dr. Reddy’s Laboratories Ltd.
is expected to mainly conduct the clinical study involving up to 1,000 people to assess its effectiveness, according to the sources.
Earlier this month, Fujifilm
said it had granted exclusive rights to Dr. Reddy’s to develop, produce and sell the potential COVID-19 treatment drug overseas, along with Dubai-based Global Response Aid.
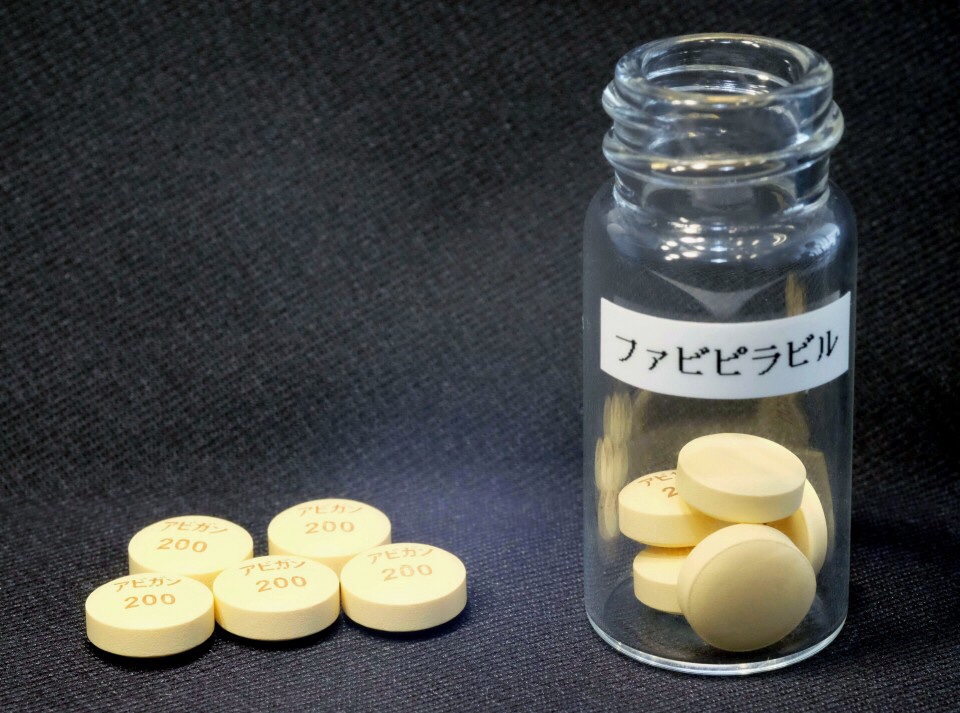
Avigan
has been seen as a possible treatment for the COVID-19 respiratory disease caused by the novel coronavirus.
Fujifilm Toyama Chemical Co., the Japanese firm’s subsidiary that developed Avigan,
also known as favipiravir, was initially planning to conduct its clinical study targeting 96 people in Japan from late March through the end of June.
But the trial did not proceed in accordance with the plan as the number of infections in Japan was dwindling some weeks during the period.
The number of COVID-19 infections in Kuwait was more than 58,200 as of Saturday, while that of Japan came to some 24,100, according to a tally by Johns Hopkins University.
The sources said if effective data were collected during the study in Kuwait, they could be used in Japan.
Earlier this month, Fujita Health University, which has led a team carrying out clinical tests of Avigan, said again its study failed to demonstrate a clear efficacy in treating coronavirus patients at an early stage of the disease.
Dr. Reddy’s partners with FUJIFILM and Global Response Aid for Avigan® (favipiravir), a potential treatment of COVID-19
https://www.drreddys.com/media/904732/press-release_dr-reddys-avigan.pdf