COVID-19: Avigan Observational Study / Interim Report: Japanese Society of Infectious Diseases HP
COVID-19:
Fujita Medical University: (Aichi Prefecture)
On May 26, published “Results of observational studies on 2158 Japanese patients” on the website of the Japanese Society of Infectious Diseases.
“In the interim report, there are still no treatment results for patients who do not use Avigan,”
Breakdown of clinical trial subjects:
Number of patients who participated in the observational study.
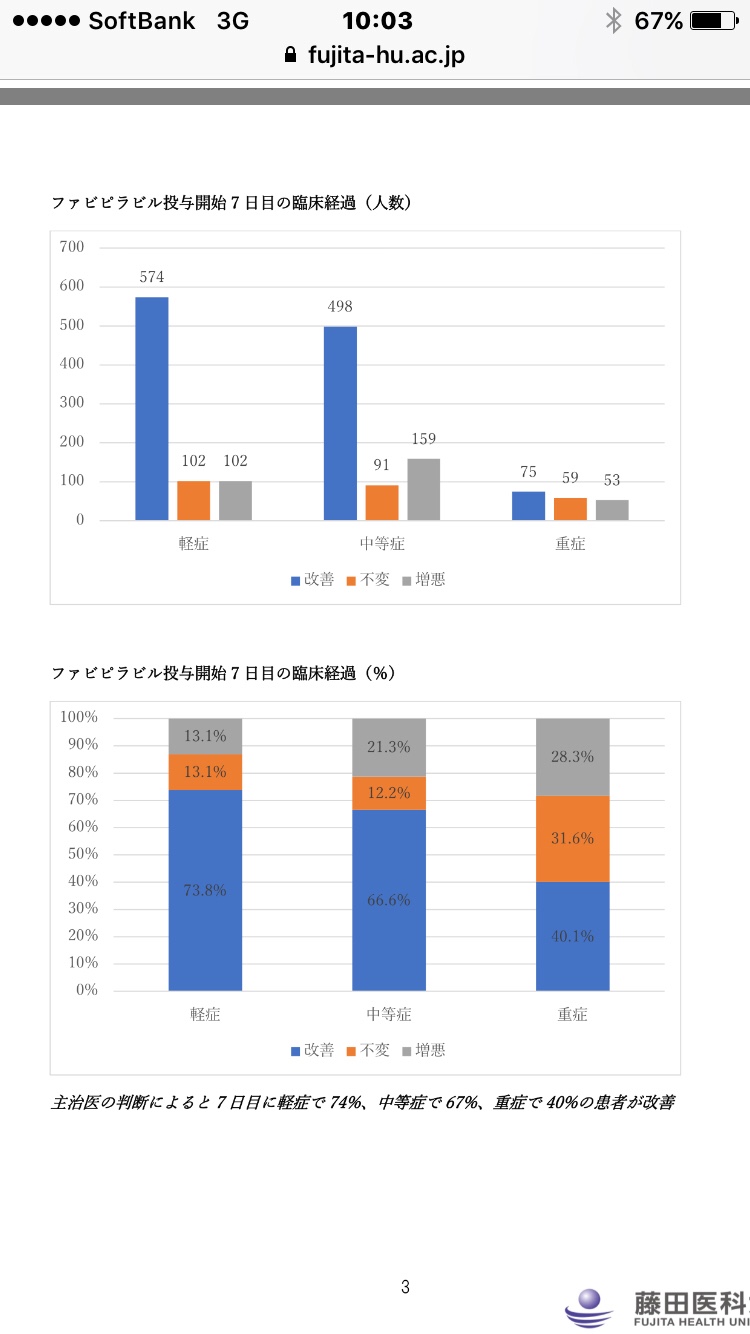
Mild patients who do not need oxygen inhalation: 976 (45%)
Intermediate patients requiring oxygen inhalation: 947 (44%)
Severely ill patients requiring ventilator: 235 (11%)
Days after administration:
Judgment is based on the number of days that have elapsed since the start of use of Avigan.
Day 7,
Day 14,
1 month after admission
summarized the symptoms of.
Analysis for 1282 people:
On day 14:
The percentage of improvement in symptoms
Mild: 88%, symptom improved.
Moderate: 85%, symptoms improved.
Severe: 60%, symptom improved.
On the other hand, one month later:
Severe: 32% of people died,
The symptoms of 5% of people have worsened.
Fujita Medical School:
Fujita Medical School has compiled the results.
This announcement is only an interim report as of May 15.
(Asahi Shimbun Digital)
https://news.yahoo.co.jp/articles/d95973550cbac4c9e82c123c87966aad87f9ddb5
Fujita Medical University, Avigan research interim report:Chemical Daily
https://www.chemicaldaily.co.jp/
Announcement of interim report on Favipiravir (Avigan) observational research
https://www.fujita-hu.ac.jp/news/j93sdv0000005nw1-att/j93sdv0000005oqn.pdf
Favipiravir observational research interim report
http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_favip_0526.pdf