Biogen Plans Regulatory Filing for Aducanumab in Alzheimer’s Disease Based on New Analysis of Larger Dataset from Phase 3 Studies
October 22, 2019
Biogen Inc.
Eisai Co., Ltd.
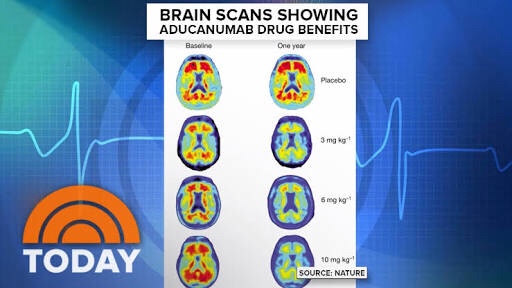
New analysis of larger dataset showed that aducanumab reduced clinical decline in patients with early Alzheimer’s disease as measured by the pre-specified primary and secondary endpoints
Based on discussions with the FDA, the Company plans to submit a Biologics License Application in early 2020
Biogen aims to offer aducanumab to eligible patients previously enrolled in clinical studies
The positive results of this new analysis were driven primarily by greater exposure to high dose aducanumab in the larger dataset as compared to data available at the time of the futility analysis
News Release:2019 | Eisai Co., Ltd.