Biogen计划根据第三阶段研究的更大数据集的新分析,制定针对阿兹海默症的Aducanumab的监管备案
十月22,2019
百健公司
卫材有限公司
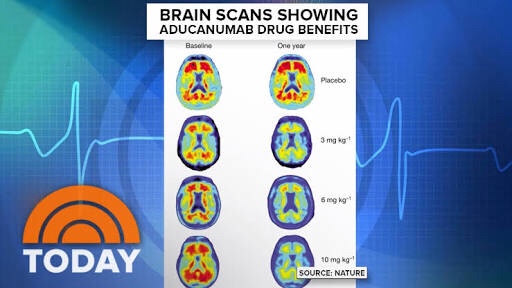
对更大数据集的新分析表明,按照预先指定的主要终点和次要终点衡量,阿德那单抗可减少阿尔茨海默氏病早期患者的临床下降
根据与FDA的讨论,公司计划在2020年初提交《生物制剂许可证》申请
Biogen旨在为先前参与临床研究的合格患者提供aducanumab
与无效分析时可用的数据相比,此新分析的积极结果主要是由更大的数据集中更大剂量的高剂量阿杜那单抗所致
新闻发布:2019 | 卫材有限公司
https://www.eisai.co.jp/news/2019/news201979.html
Biogen Plans Regulatory Filing for Aducanumab in Alzheimer’s Disease Based on New Analysis of Larger Dataset from Phase 3 Studies
October 22, 2019
Biogen Inc.
Eisai Co., Ltd.
New analysis of larger dataset showed that aducanumab reduced clinical decline in patients with early Alzheimer’s disease as measured by the pre-specified primary and secondary endpoints
Based on discussions with the FDA, the Company plans to submit a Biologics License Application in early 2020
Biogen aims to offer aducanumab to eligible patients previously enrolled in clinical studies
The positive results of this new analysis were driven primarily by greater exposure to high dose aducanumab in the larger dataset as compared to data available at the time of the futility analysis
News Release:2019 | Eisai Co., Ltd.