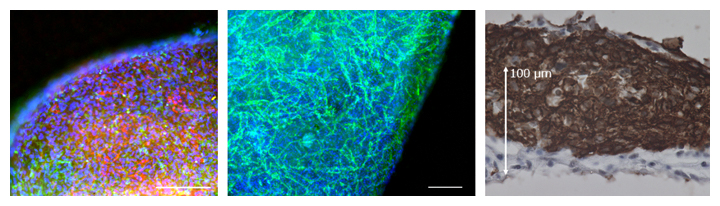
Figure 1 Three-dimensional microheart tissue from human iPS cells obtained by dynamic training culture
(Left) Cardiomyocytes (red) and vascular parietal cells (cells surrounding the vascular endothelium; green). The scale bar is 200 μm.
(Middle) Vascular endothelial cells (green). A vascular network is formed in the microheart tissue. The scale bar is 200 μm.
(Right) Myocardium (brown). Microcardiac tissue has a thickness of more than 150 μm.
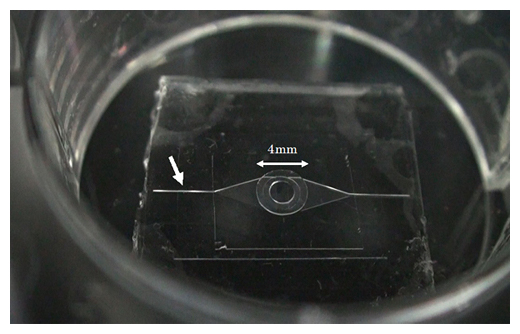
Fig. 2 Microfluidic chip manufactured by MEMS technology
The central circle (4 mm in diameter) has a convex part with a diameter of 2 mm at the bottom, and when the micro heart is placed on top, its pulsation is transmitted to the micro channel below. The white arrow indicates a groove with a width of 100 μm on the inflow side of the microchannel through which fluorescent particles flow.
RIKEN: “Heart-on-chip” with iPS cells: Developed microdevices
RIKEN:
Collaborative research group:
Human iPS cell technology and
Using microdevice technology,
Developed “Heart-on-chip [4] type micro device”.
The Heart-on-Chip [4] microdevice is a highly sensitive evaluation of human heart function.
Results of this research:
The results of this research will contribute to cardiac regenerative medicine and drug discovery research using iPS cells.
Regenerative medicine for heart disease:
In regenerative medicine and drug discovery research for heart disease, the application of three-dimensional human heart tissue artificially produced from human iPS cells is expected.
Functional evaluation of artificial heart tissue:
Traditional problem:
A system that can evaluate the function of the reproduced artificial heart tissue with high sensitivity has not been established.
This development:
This time, the joint research group has developed a “heart-on-chip type microdevice” and established an unprecedented high-sensitivity artificial heart function evaluation system.
Cell sheet 3D microcardiac tissue:
From human iPS cells:
Developed a cell sheet-like three-dimensional microheart tissue thickened by dynamic training culture [5].
Using microfabrication technology:
A combination of fluid cardiac function evaluation systems using microchannels.
This research will be published in the online version of the scientific journal “Scientific Reports” (November 5th).
RIKEN
https://www.riken.jp/press/2020/20201105_5/
Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function
Abstract
Human iPS cell (iPSC)-derived cardiomyocytes (CMs) hold promise for drug discovery for heart diseases and cardiac toxicity tests.
To utilize human iPSC-derived CMs,
the establishment of three-dimensional (3D) heart tissues from iPSC-derived CMs and other heart cells,and a sensitive bioassay system to depict physiological heart function are anticipated.
We have developed a heart-on-a-chip microdevice (HMD)
as a novel system consisting of dynamic culture-based 3D cardiac microtissues
derived from human iPSCs and microelectromechanical system (MEMS)-based microfluidic chips.The HMDs
could visualize the kinetics of cardiac microtissue pulsations by monitoring particle displacement,which enabled us to quantify the physiological parameters, including fluidic output, pressure, and force.
The HMDs
demonstrated a strong correlation between particle displacement and the frequency of external electrical stimulation.The transition patterns
were validated by a previously reported versatile video-based system to evaluate contractile function.The patterns
are also consistent with oscillations of intracellular calcium ion concentration of CMs, which is a fundamental biological component of CM contraction.The HMDs showed a pharmacological response to isoproterenol,
a β-adrenoceptor agonist, that resulted in a strong correlation between beating rate and particle displacement.
Thus, we have validated the basic performance of HMDs as a resource for human iPSC-based pharmacological investigations.
Scientific Reports