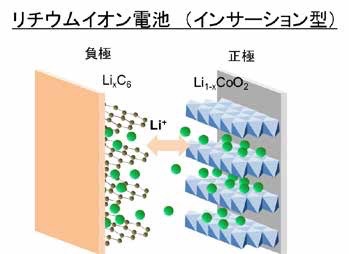
Figure 1 Conceptual diagram of lithium-ion battery (insertion type)
(Example) 3Li+FeF3 ⇄ 3LiF+Fe (Example) 2LaF3 + 3Cu ⇄ 2La + 3CuF2 4Li+a-TiS4 ⇄ Li4TiS4
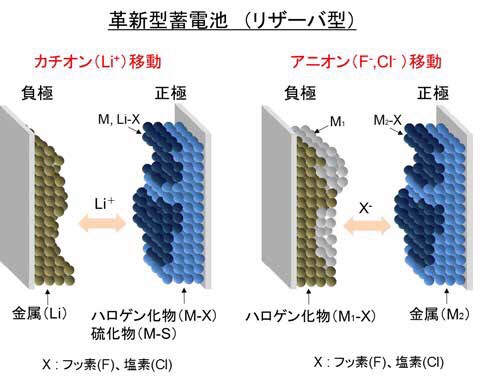
Figure 2 Conceptual diagram of innovative storage battery (reservoir type)
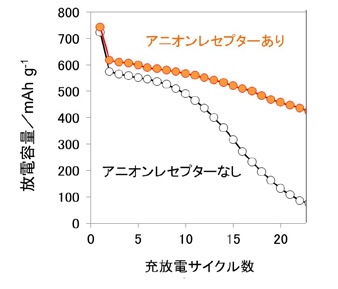
Figure 3 Verification of effect of anion receptor on cycle characteristics of metal fluoride electrode (FeF3)
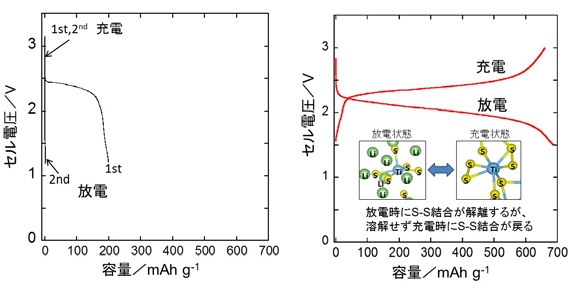
Figure 4 Charge/discharge behavior of sulfur electrode (left) and amorphous metal sulfide electrode (right)
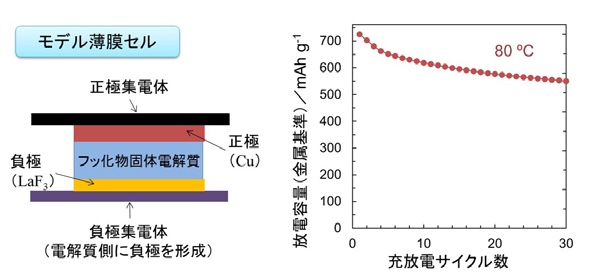
Figure 5 Schematic diagram and cycle characteristics of a fluoride all-solid-state thin film cel
Kyoto Univ/Toyota: “Fluoride ion battery”: EV running 1000 km
Kyoto University/Toyota:
From Tokyo to Fukuoka with one charge.
An EV running over 1,000 kilometers is under development to realize it.
“Fluoride ion battery”:
The “fluoride ion battery” has made a name for itself as a promising candidate for a storage battery that surpasses the current lithium-ion battery.
Kyoto University and Toyota:
Prototype prototype of “fluoride ion battery”.
We have set a goal of “enhancing electricity storage performance by a factor of 7 compared to lithium-ion batteries.”
It’s too early to think that an answer has been given, but researchers around the world are in the process of finding a solution.
Nihon Keizai Shimbun
https://r.nikkei.com/article/DGXMZO62404420X00C20A8TJM000?n_cid=NMAIL007_20200807_H&s=4
Building basic technology for innovative storage batteries that surpass lithium-ion batteries
-Announcement of results of RISING project-
-SPring-8: High energy X-ray diffraction-
Metal fluoride electrode: solving the problem (Fig. 3)
For metal fluoride electrodes that insert and desorb a large amount of lithium ions,
Since the lithium fluoride produced is solid, it has a low charge acceptability.
Therefore, an additive that allows this lithium fluoride to dissolve in the electrolyte solution,
Specifically, add anion receptor *2 that binds to fluorine as an additive
Has succeeded in greatly improving the cycle characteristics.
Short life of sulfur electrode: solving the problem
Similarly, “a sulfur electrode that reacts with a large amount of lithium ions has conventionally been discharged.
There was a problem that sulfur was dissolved by electricity and the life was short.”
By “immobilizing sulfur as an amorphous metal sulfide that is covalently bonded to a metal,” stable charge and discharge became possible.
Large synchrotron radiation facility SPring-8: High energy X-ray diffraction (Fig. 4)
The reaction mechanism was clarified by applying high-energy X-ray diffraction*4 at the large synchrotron radiation facility SPring-8*3.
It was found that the bond between sulfur atoms is formed/dissociated by charge/discharge.
As typified by the reaction mechanism analysis of amorphous materials, we have made great strides in elucidating the phenomena that occur in batteries, which was difficult in the past, using advanced analysis techniques such as synchrotron radiation and neutrons.
Proposed halide storage battery:
We also paid attention to “migration of negatively charged halide ions”.
We also worked on verification of its operation by proposing “a halide storage battery capable of multi-electron transfer”.
If the product is a chloride system that is too soluble in the electrolyte,
Resolved by increasing the concentration of electrolytes and electrolyte salts with low solubility,
Developed “Technology to control ion migration at the electrode-electrolyte interface at the nano level”.
Achieved high utilization and long life:
This method is also used to suppress the dissolution of zinc species that are too soluble in the electrolyte in aqueous zinc-air batteries, and has achieved unprecedented high utilization and long life.
All-solid-state battery: Ions are exchanged between solids without using a solution (Fig. 5)
In the case of fluoride, a solid fluoride electrolyte with high ionic conductivity was constructed using a model thin film cell.
By using an all-solid-state battery*5 that exchanges ions between solids without passing through a solution, we have succeeded in activating materials that were previously inactive, and have shown that they show high charge/discharge capacity.
This research:
A system that has been thought to be difficult to use in the past was demonstrated by “a concept such as solubility control” that “an innovative storage battery with an energy density of 500Wh/kg and high energy density that far exceeds LIB can be constructed.”
http://www.kyoto-u.ac.jp/ja/research/research_results/2015/documents/160328_1/01.pdf