JAEA: Developed biodegradable, high-strength gel: From citric acid, water, etc.
Japan Atomic Energy Agency (JAEA),
Tokyo Metropolitan Industrial Technology Research Center (Tokyo Metropolitan Industrial Technology Research Institute),
University of Tokyo
On October 30, it announced that it had succeeded in developing an environmentally friendly high-strength gel material “freeze-crosslinked cellulose nanofiber gel”.
With “cellulose nanofiber” of wood
Citric acid contained in lemon,
And it is composed of water.
Cellulose nanofibers:
“Carboxymethyl cellulose (CMC) nanofiber” is a highly safe material that is also used as a food additive and a thickener for cosmetics.
The molecule has a “carboxyl group” which is one of the reactive functional groups of organic compounds.
It can be reacted with other substances to make gels and films.
This study:
CMC nanofibers and citric acid will be used in the experiment.
In an environment of -20 ° C
In frozen CMC nanofiber solution
Mix the citric acid solution,
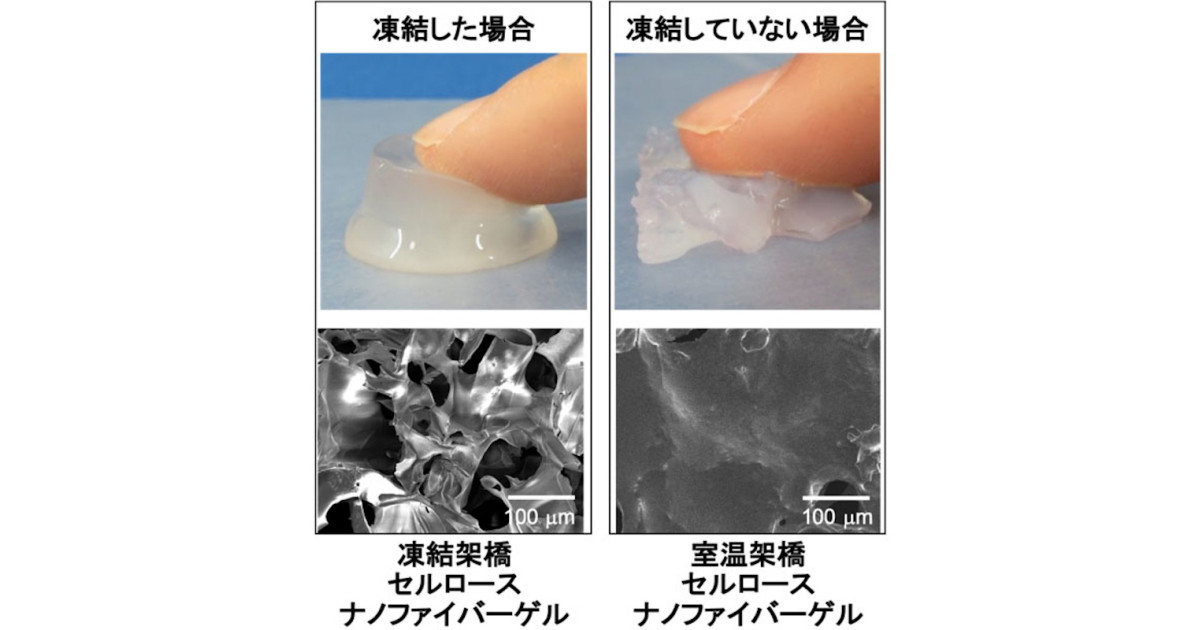
Melted in an environment of -4 ° C.
As a result, the white and opaque hydrogel as soon as the ice melts,
The formation of freeze-crosslinked CNF gel was confirmed.
As a result of examining the water retention of the freeze-crosslinked CNF gel,
It turns out that “about 95% of the total weight of water can be contained inside the gel.”
Details were published in the online version of the international academic journal “ACS Applied Polymer Materials”.
Mynavi News
https://news.mynavi.jp/article/20201104-1452145/
Eco-friendly Carboxymethyl Cellulose Nanofiber Hydrogels Prepared via Freeze Cross-Linking and Their Applications
Abstract
We developed a cross-linking method using freeze concentration and used it to synthesize
a carboxymethyl cellulose nanofiber (CMCF) hydrogel
with high water content (>94%),
high compressive strength (>80 MPa),
and high compressive recoverability.
The hydrogels
were prepared by adding an aqueous solution of citric acid (CA) to a frozen CMCF sol and then thawing the sol.The reaction between the freeze-concentrated CMCF and CA created a rigid porous structure with a pore diameter of approximately 80 μm, which resembled the ice crystal structure.
The stress–strain curves of the hydrogel during repeated compression up to 80% strain
were similar over three cycles, which indicated that their cross-linked structure had high stability to compressive stress.
ACS Applied Polymer Materials