COVID-19: The Univ of Tokyo, Nafamostat mesylate (Fusan) : Prevents Virus Invasion, Clinical Trial
COVID-19: Tokyo University
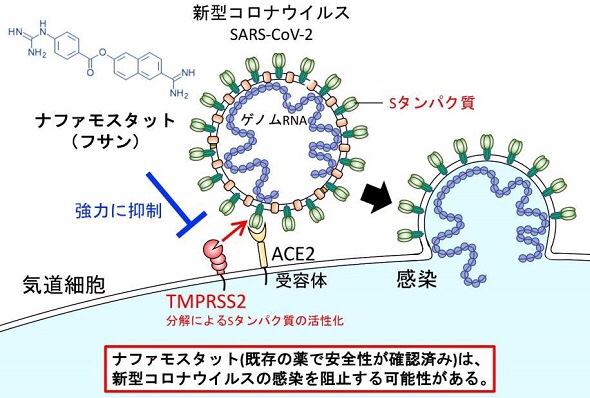
Nafamostat mesylate (trade name Fusan) was identified as a drug that blocks the virus entry process.
Nafamostat mesylate prevents SARS-CoV-2 infection / initial fusion of the viral outer membrane with the cell membrane of the infected cell.
Germany: Research Group (1)
Earlier this March, the German group announced the efficacy of Camostat mesylate (trade name Foypan) against SARS-CoV-2 (Ref. 1).
Nafamostat: results of administration
Nafamostat blocked the virus invasion process at less than one-tenth the concentration compared to camostat.
Both nafamostat and camostat are being developed in Japan as a treatment for acute pancreatitis.
A drug that has been prescribed for many years in Japan.
Sufficient clinical data has been accumulated for safety, and clinical trials can be conducted promptly.
The University of Tokyo Institute of Medical Science
https://www.ims.u-tokyo.ac.jp/imsut/jp/about/press/page_00060.html
Identification of an existing Japanese pancreatitis drug, Nafamostat, which is expected to prevent the transmission of new coronavirus infection (COVID-19) March 23, 2020
Nafamostat mesylate (brand name: Fusan), which is the drug used to treat acute pancreatitis, may effectively block the requisite viral entry process the new coronavirus (SARS-CoV-2) uses to spread and cause disease (COVID-19). The University of Tokyo announced these new findings on March 18, 2020.
According to the new research, Nafamostat can prevent the fusion of the envelope of the virus with host cell surface membranes, the first step in infection with the causative virus SARS-CoV-2. Nafamostat can inhibit the membrane fusion at a concentration less than one-tenth that of Camostat mesylate (brand name: Foypan), which was recently identified by a German group as an inhibitor of SARS-CoV-2 infection (Reference 1).
Both Nafamostat and Camostat were developed in Japan as treatments for pancreatitis and some other diseases. These drugs have been prescribed in Japan for many years and have adequate clinical data with regard to safety.
The University of Tokyo plans to launch clinical trials in April 2020 in order to evaluate the effectiveness of these two drugs for treating COVID-19.
THE INSTITUTE OF MEDICAL SCIENCE, THE UNIVERSITY OF TOKYO
https://www.ims.u-tokyo.ac.jp/imsut/en/about/press/page_00002.html