COVID-19: Development of New Corona Therapeutics: Participation in Global Joint Trials
February 28, 2020 11:00
Japan: National Center for Global Health and Medicine
The National Center for Global Health and Medicine is examining the efficacy of therapeutic agents with three candidates.
United States: National Institutes of Health (NIH)
NIH is leading an international joint clinical trial of an unapproved drug for Ebola.
Japan will also participate in this from March.
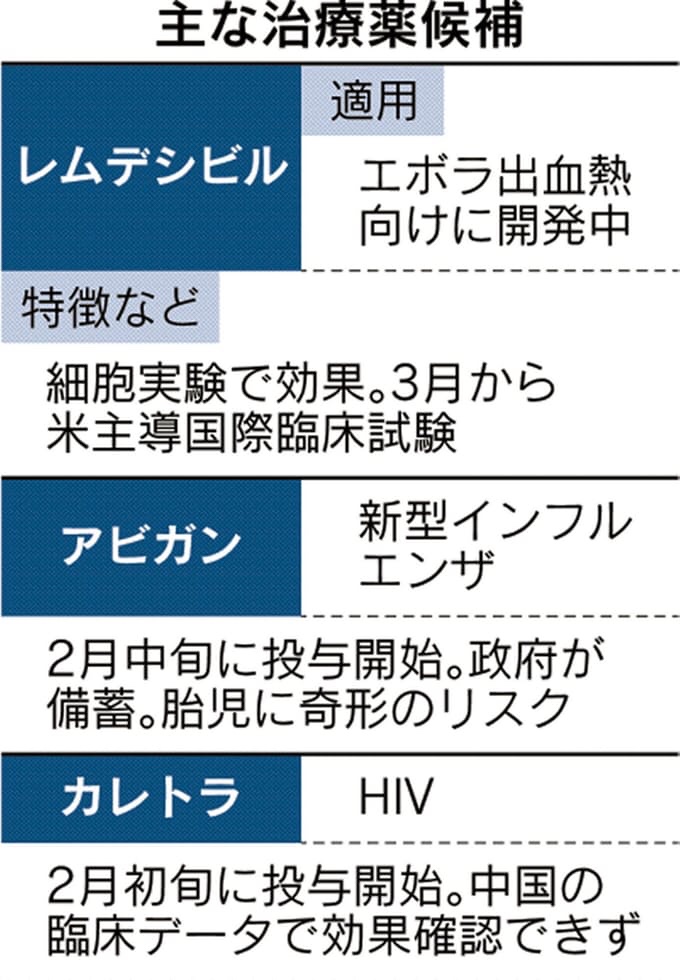
Ebola hemorrhagic fever: remdesivir
The Japanese government will participate in an international clinical trial of remdesivir, a treatment for Ebola.
NIH conducts clinical trials on 400 subjects at 50 locations worldwide.
In Japan, administration as an observational study will be started in parallel.
Avigan / Kaletra
The efficacy of the following therapies is being verified along with remdesivir.
Dosage administration to patients has also started in Japan.
Avigan:
There is already a stockpile.
The government has stockpiled approximately 2 million people in anticipation of a pandemic influenza pandemic.
Its strength is that it can easily respond to the increase in the number of patients.
However, it is known that fetal malformations occur in animal experiments, and its use during pregnancy is contraindicated in the treatment of new influenza.
Kaletra:
It has been used for HIV treatment for a long time and its safety is high.
However, according to the Shanghai Public Health Clinical Center / latest clinical data, there was no evidence of a recovery-promoting effect when administered to patients with new-type pneumonia.
Nihon Keizai Shimbun
https://r.nikkei.com/article/DGXMZO56164610Y0A220C2EA2000
https://www.google.co.jp/amp/s/www.sankei.com/life/amp/200321/lif2003210052-a.html