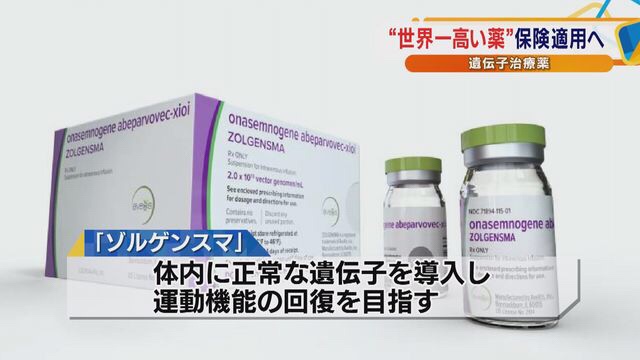

Japan: Gene therapy drug “Solgensma” approved: 200 million yen per dose
Japan Ministry of Health, Labor and Welfare: Special Working Group
On February 26, the Ministry of Health, Labor and Welfare / Special Committee approved “Sales of the world’s highest drug in Japan”.
Production and sale of Sorgensma, a spinal muscular atrophy / gene therapy drug, is licensed in Japan.
It is expected to be officially approved in March.
Spinal muscular atrophy:
Spinal muscular atrophy is an intractable disease that causes muscle atrophy and dyspnea by about half a year after birth.
This is the second gene therapy drug in Japan.
Pharmaceutical giant: Novartis Pharma
Pharmaceutical giant Novartis Pharma has applied.
In the United States, the Food and Drug Administration (FDA) approved it last May.
A single dose ends treatment, but costs in the United States are more than 200 million yen.
May this year: insurance applied
With approval in Japan, insurance will be applied in May this year.
High drug prices can affect the funding of public health insurance.
Society (TOKYO Web)