COVID-19: PCR test of Takara Bio, 5000 cases in 2 hours: Urgent application to US FDA
COVID-19:
Takara Bio USA:
On June 9, a US subsidiary announced, “We have developed a method that significantly increases the efficiency of PCR tests for the presence of new coronavirus infection.”
A maximum of 5,000 cases can be inspected in 2 hours.
He is applying for an emergency use license with the US FDA and is expected to be approved by the end of June.
New PCR test method:
It was jointly developed by Takara Bio’s US subsidiary (Takara Bio USA) and bioSyntagma by combining existing equipment and reagents.
Compared with the current mainstream method in the US, it can significantly speed up inspection.
BioSyntagma owns the rights to this method.
Takara Bio said, ” not thinking about expanding it in Japan.”
Kyoto Shimbun
https://www.kyoto-np.co.jp/articles/-/272636
Development of high-throughput PCR assay for new coronavirus
Takara Bio USA, Inc., a US subsidiary of Takara Bio Co., Ltd. (TB USA, California, USA)
On June 8, 2020 (local time), we made a press release on the development of a high-throughput PCR assay for the new coronavirus.
https://ir.takara-bio.co.jp/ja/news_all/news_IR/auto_20200610441125/pdfFile.pdf
Takara Bio USA, Inc. and bioSyntagma, Inc. develop method for large-scale automated COVID-19 testing
DATE: June 8, 2020
AUTHOR: Takara Bio USA, Inc.
CATEGORIES: Press release
Mountain View, CA—
June 8, 2020—
Takara Bio USA, Inc. (TBUSA), a pioneering life science instrument and reagent company and wholly owned subsidiary of Takara Bio Inc.,
collaborated with bioSyntagma, Inc. and their partners to develop and validate a new high-throughput method for detecting SARS-CoV-2.
The method
employs automation technology and reagents from TBUSA to detect viral RNA via real-time PCR and will enable rapid, large-scale testing of thousands of patient samples per day.
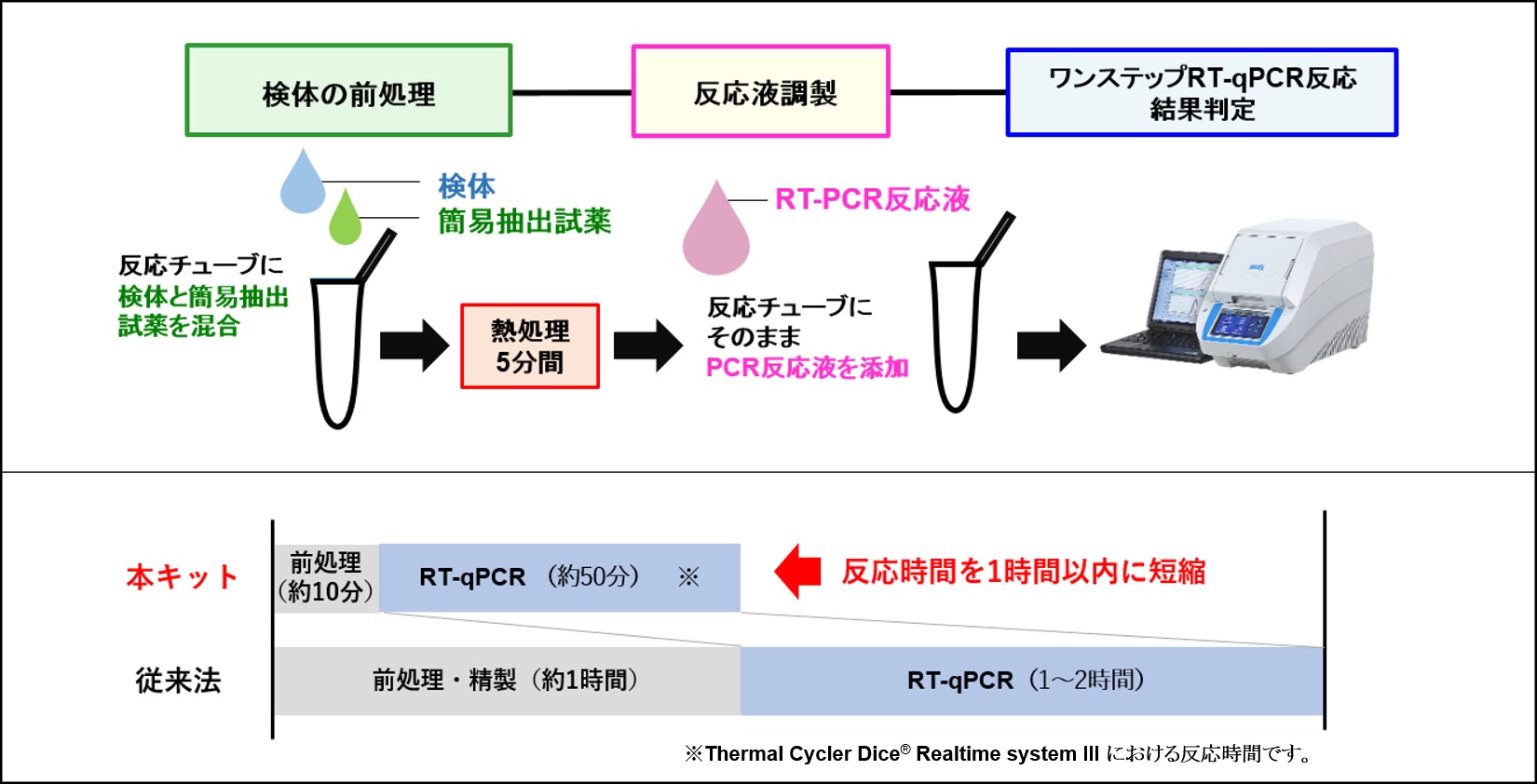
The method uses TBUSA’s SmartChip real-time PCR instrument, chips, and reagents to run 5,184 reactions per chip in less than 30 minutes of direct hands-on time.
Each reaction is at nanoliter scale, which reduces variability via elimination of the standard preamplification step and reduces costs via decreased reagent volume.
The trusted SmartChip Real-Time PCR System is already widely used for detection of antibiotic and antimicrobial resistance around the world, and is ideally suited to address the major need for rapid and accurate SARS-CoV-2 detection.
President of TBUSA Carol Lou states,
As shelter-in-place orders are lifted, controlling the COVID-19 pandemic will depend on our ability to detect SARS-CoV-2 from a large number of samples with precision, reproducibility, and speed.
To support this effort,
we optimized our existing chemistries and developed SmartChip protocols that maximize the number of samples processed while minimizing costs.
The work we have accomplished with bioSyntagma, plus their partners’ further development of diagnostic tests based on our work, will contribute to comprehensive and faster detection of COVID-19.
Scottsdale-based bioSyntagma
is a biotech spinoff of Arizona State University and serves as the development and validation partner of P2 Diagnostics, LLC.
The new SmartChip testing method
will be adopted by this and other molecular testing labs that are certified by CLIA (Clinical Laboratory Improvement Amendments) and therefore eligible to develop and perform COVID-19 diagnostic tests.
“The rapid development of this novel COVID-19 detection method
was made possible through a highly productive collaboration between bioSyntagma and TBUSA,
and the results of this effort will soon have an impact on the ability to detect SARS-CoV-2 in nasal and saliva samples from many patients,
said David Richardson, CEO of bioSyntagma.
bioSyntagma and partners
are seeking an Emergency Use Authorization (EUA) from the FDA for COVID-19 detection using the TBUSA SmartChip method.
Ipsum Diagnostics, LLC and Hackensack University Medical Center—two of TBUSA’s customers in the US—
have already obtained EUAs for their COVID-19 tests using Takara Bio’s one-step RT-PCR reagents.