COVID-19:Chugai 扩大了抗体鸡尾酒疗法的适应症:Lonapreve™
2021 年 10 月 11 日
中外:
– 抗体鸡尾酒疗法,Lonapreve™
随着新的冠状病毒感染,
我们申请扩大适应症为“无症状感染者的预防和治疗药物”。
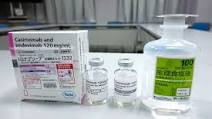
单克隆抗体
Lonapreve™
抗 SARS-CoV-2 单克隆抗体“Lonapreve™”[casilibimab / imdebimab]
“预防 COVID-19”和
用于“无症状感染者的治疗”
我们很高兴地通知您,我们已向厚生劳动省提交了扩大适应症的申请。
皮下给药添加:
配合本次扩大适应
除了经批准的静脉给药
我申请了额外的用途以启用皮下给药。
在此批准申请中,我们想申请特殊批准。
应用新闻发布 | 中外
https://www.chugai-pharm.co.jp/news/detail/20211011170001_1146.html
Chugai seeks expanded use of antibody cocktail
Chugai Pharmaceutical
on Monday asked Japan’s health ministry to greenlight the expanded use of a COVID-19 antibody cocktail.
The treatment
is currently authorized for use in patients with mild to moderate symptoms who are at risk of falling gravely ill.
Chugai, which has the exclusive rights to sell the drug in Japan,
wants it approved for use in people without symptoms and as a measure to prevent infection.
The cocktail of two virus-neutralizing antibodies is administered intravenously.
About 35,000 people in Japan
are believed to have undergone the treatment as of October 5.Chugai says a global clinical study has shown that
the drug reduced the risk of asymptomatic COVID-19 patients progressing to symptomatic by 31 percent.
The study also included family members who were not infected at the start of the trial and remained in close contact with the patients.
Chugai says the drug reduced their risk of symptomatic infection by 81 percent.
NHK WORLD-JAPAN News