COVID-19:AGCがPfizer-BioNTech Vaccineを製造:プラスミドDNA(動画):
COVID-19: AGC manufactures Pfizer-BioNTech Vaccine: plasmid DNA:
COVID-19:AGC 生产 Pfizer-BioNTech 疫苗:质粒 DNA
AGC Biologics’ Heidelberg:
6月8日、AGC Biologicsが、「Pfizer-BioNTech COVID-19 Vaccineの原材料であるプラスミドDNAの製造」を受託した。
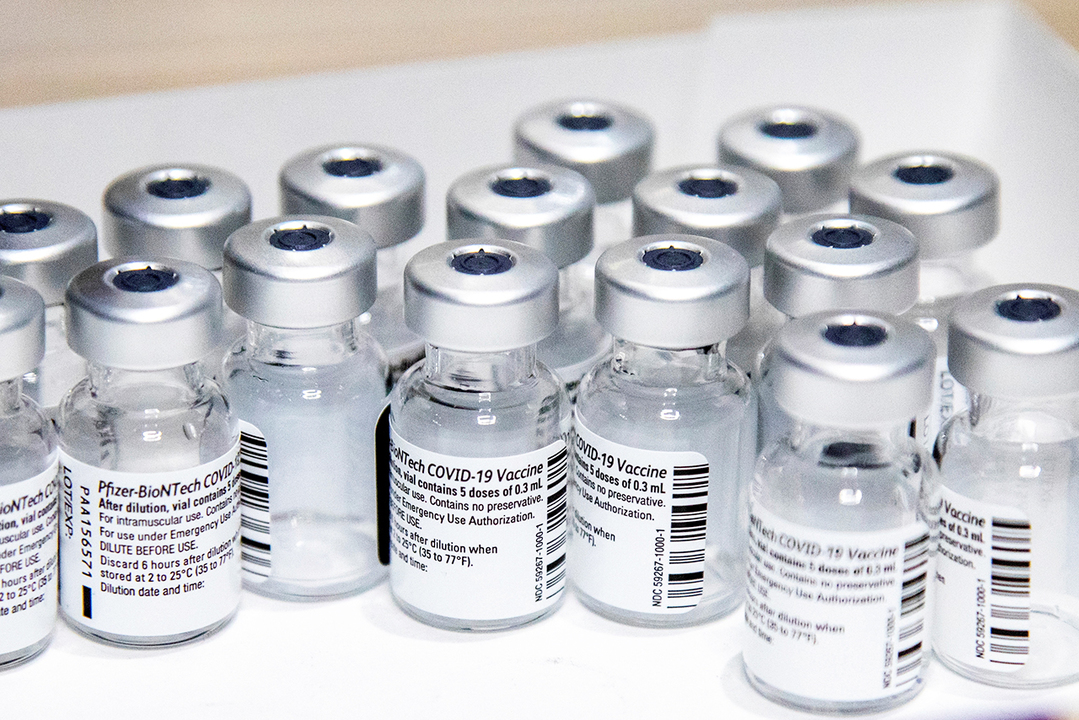
Pfizer-BioNTech COVID-19 Vaccine:(日本名:コミナティ)
コミナティは、米ファイザーと独ビオンテックが共同開発したワクチン。
いわゆる「mRNAワクチン」だ。
日本で特例承認済み:
- 日本では、
- 2021年2月、国内初のワクチンとして、
- Pfizer-BioNTech COVID-19 Vaccineが特例承認された。
6月7日段階で1600万回以上の接種が完了している。
2021年中に、ファイザー・ビオンテック製ワクチンが、1億9400万回分供給される。
Business Insider Japan
https://www.businessinsider.jp/post-236366
AGC Biologics’ Heidelberg Facility to Further Supply Plasmid DNA for COVID-19 Vaccine
AGC (Headquarters: Tokyo), a world-leading manufacturer of glass, chemicals, and high-tech materials,
has announced that its biopharmaceutical CDMO subsidiary, AGC Biologics (Headquarters: United States), has announced its partnership with BioNTech SE (Nasdaq BNTX)
to further supply Plasmid DNA (pDNA) starting material for the Pfizer-BioNTech COVID-19 vaccine, at AGC’s Heidelberg, Germany facility.
We are honored that
BioNTech has entrusted us to become part of their global supply network in an effort to make the vaccine available to as many people as possible around the world,” says AGC Biologics Chief Business Officer, Mark Womack.
We are very proud of our efforts
to provide our global customers with the essential materials needed to rapidly deliver vaccines in the fight against the COVID-19 pandemic.
AGC Biologics
will manufacture and supply BioNTech with pDNA starting material, an essential component of BioNTech’s mRNA-based vaccine manufacturing process.
AGC Biologics’ Heidelberg facility
has over 20 years of experience delivering a very wide range of microbial programs for our customers.
In addition,
the site is AGC’s Center of Excellence for Plasmid DNA (pDNA) production, as part of their end-to-end Cell and Gene Therapy offering.
The Pfizer-BioNTech COVID-19 Vaccine, which is based on BioNTech proprietary mRNA technology,
was developed by both BioNTech and Pfizer.
BioNTech is the Marketing Authorization Holder in the European Union,
and the holder of emergency use authorizations or equivalents
in the United States (jointly with Pfizer), United Kingdom,
Canada and other countries
in advance of a planned application for full marketing authorizations in these countries.
News | AGC News | AGC